Preparation of human decellularized peripheral nerve allograft using amphoteric detergent and nuclease
- PMID: 33510098
- PMCID: PMC8328754
- DOI: 10.4103/1673-5374.306091
Preparation of human decellularized peripheral nerve allograft using amphoteric detergent and nuclease
Abstract
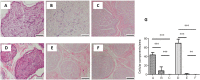
Animal studies have shown that amphoteric detergent and nuclease (DNase I and ribonuclease A) is the most reliable decellularization method of the peripheral nerve. However, the optimal combination of chemical reagents for decellularization of human nerve allograft needs further investigation. To find the optimal protocol to remove the immunogenic cellular components of the nerve tissue and preserve the basal lamina and extracellular matrix and whether the optimal protocol can be applied to larger-diameter human peripheral nerves, in this study, we decellularized the median and sural nerves from the cadavers with two different methods: nonionic and anionic detergents (Triton X-100 and sodium deoxycholate) and amphoteric detergent and nuclease (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), deoxyribonuclease I, and ribonuclease A). All cellular components were successfully removed from the median and sural nerves by amphoteric detergent and nuclease. Not all cellular components were removed from the median nerve by nonionic and anionic detergent. Both median and sural nerves treated with amphoteric detergent and nuclease maintained a completely intact extracellular matrix. Treatment with nonionic and anionic detergent decreased collagen content in both median and sural nerves, while the amphoteric detergent and nuclease treatment did not reduce collagen content. In addition, a contact cytotoxicity assay revealed that the nerves decellularized by amphoteric detergent and nuclease was biocompatible. Strength failure testing demonstrated that the biomechanical properties of nerves decellularized with amphoteric detergent and nuclease were comparable to those of fresh controls. Decellularization with amphoteric detergent and nuclease better remove cellular components and better preserve extracellular matrix than decellularization with nonionic and anionic detergents, even in large-diameter human peripheral nerves. In Korea, cadaveric studies are not yet legally subject to Institutional Review Board review.
Keywords: detergent; human decellularized nerve graft; median nerve; nuclease; sural nerve.
Conflict of interest statement
None
Figures






Similar articles
-
Comparison of systematically combined detergent and nuclease-based decellularization methods for acellular nerve graft: An ex vivo characterization and in vivo evaluation.J Tissue Eng Regen Med. 2019 Jul;13(7):1241-1252. doi: 10.1002/term.2874. Epub 2019 Jun 14. J Tissue Eng Regen Med. 2019. PMID: 31050871
-
Novel Sodium Deoxycholate-Based Chemical Decellularization Method for Peripheral Nerve.Tissue Eng Part C Methods. 2020 Jan;26(1):23-36. doi: 10.1089/ten.TEC.2019.0135. Epub 2019 Dec 19. Tissue Eng Part C Methods. 2020. PMID: 31724493
-
Development of a decellularization method to produce nerve allografts using less invasive detergents and hyper/hypotonic solutions.J Plast Reconstr Aesthet Surg. 2016 Dec;69(12):1690-1696. doi: 10.1016/j.bjps.2016.08.016. Epub 2016 Sep 8. J Plast Reconstr Aesthet Surg. 2016. PMID: 27697539
-
Decellularization of human dermis using non-denaturing anionic detergent and endonuclease: a review.Cell Tissue Bank. 2015 Jun;16(2):249-59. doi: 10.1007/s10561-014-9467-4. Epub 2014 Aug 28. Cell Tissue Bank. 2015. PMID: 25163609 Free PMC article. Review.
-
Nerve Repair Using Decellularized Nerve Grafts in Rat Models. A Review of the Literature.Front Cell Neurosci. 2018 Nov 19;12:427. doi: 10.3389/fncel.2018.00427. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30510503 Free PMC article. Review.
Cited by
-
Nerve regeneration using decellularized tissues: challenges and opportunities.Front Neurosci. 2023 Oct 19;17:1295563. doi: 10.3389/fnins.2023.1295563. eCollection 2023. Front Neurosci. 2023. PMID: 37928728 Free PMC article. Review.
-
Large three-dimensional cell constructs for tissue engineering.Sci Technol Adv Mater. 2021 Aug 11;22(1):571-582. doi: 10.1080/14686996.2021.1945899. eCollection 2021. Sci Technol Adv Mater. 2021. PMID: 34408551 Free PMC article. Review.
-
Decellularised extracellular matrix-based injectable hydrogels for tissue engineering applications.Biomater Transl. 2024 Jun 28;5(2):114-128. doi: 10.12336/biomatertransl.2024.02.003. eCollection 2024. Biomater Transl. 2024. PMID: 39351160 Free PMC article. Review.
-
Peripheral Nerve Decellularisation Protocol for Allogeneic Transplantation: From Tissue Procurement to Banking.Int J Mol Sci. 2025 Aug 17;26(16):7937. doi: 10.3390/ijms26167937. Int J Mol Sci. 2025. PMID: 40869258 Free PMC article.
-
Optimized Decellularization Protocol for Large Peripheral Nerve Segments: Towards Personalized Nerve Bioengineering.Bioengineering (Basel). 2022 Aug 24;9(9):412. doi: 10.3390/bioengineering9090412. Bioengineering (Basel). 2022. PMID: 36134958 Free PMC article.
References
-
- Beris A, Gkiatas I, Gelalis I, Papadopoulos D, Kostas-Agnantis I. Current concepts in peripheral nerve surgery. Eur J Orthop Surg Traumatol. 2019;29:263–269. - PubMed
-
- Boriani F, Fazio N, Fotia C, Savarino L, Nicoli Aldini N, Martini L, Zini N, Bernardini M, Baldini N. A novel technique for decellularization of allogenic nerves and in vivo study of their use for peripheral nerve reconstruction. J Biomed Mater Res A. 2017;105:2228–2240. - PubMed
-
- Du L, Wu X, Pang K, Yang Y. Histological evaluation and biomechanical characterisation of an acellular porcine cornea scaffold. Br J Ophthalmol. 2011;95:410–414. - PubMed
-
- Evans PJ, Mackinnon SE, Levi AD, Wade JA, Hunter DA, Nakao Y, Midha R. Cold preserved nerve allografts: changes in basement membrane, viability, immunogenicity, and regeneration. Muscle Nerve. 1998;21:1507–1522. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

