Altered offspring neurodevelopment in an arginine vasopressin preeclampsia model
- PMID: 33510137
- PMCID: PMC7844013
- DOI: 10.1038/s41398-021-01205-0
Altered offspring neurodevelopment in an arginine vasopressin preeclampsia model
Abstract
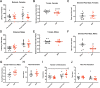
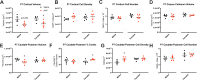
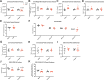
Preeclampsia is a severe gestational hypertensive condition linked to child neuropsychiatric disorders, although underlying mechanisms are unclear. We used a recently developed, clinically relevant animal model of preeclampsia to assess offspring. C57BL/6J mouse dams were chronically infused with arginine vasopressin (AVP) or saline (24 ng/h) throughout pregnancy. Adult offspring were behaviorally tested (Y-maze, open field, rotarod, social approach, and elevated plus maze). Offspring brain was assessed histologically and by RNA sequencing. Preeclampsia-exposed adult males exhibited increased anxiety-like behavior and social approach while adult females exhibited impaired procedural learning. Adult AVP-exposed males had reduced total neocortical volume. Adult AVP-exposed females had increased caudate-putamen volume, increased caudate-putamen cell number, and decreased excitatory synapse density in hippocampal dentate gyrus (DG), CA1, and CA3. At postnatal day 7 (P7), AVP-exposed male and female offspring both had smaller neocortex. At P7, AVP-exposed males also had smaller caudate-putamen volume, while females had increased caudate-putamen volume relative to neocortical size. Similar to P7, E18 AVP-exposed offspring had smaller dorsal forebrain, mainly in reduced intermediate, subventricular, and ventricular zone volume, particularly in males. Decreased volume was not accounted for by cell size or cerebrovascular vessel diameter changes. E18 cortical RNAseq revealed 49 differentially-expressed genes in male AVP-exposed offspring, over-representing cytoplasmic translation processes. In females, 31 genes were differentially-expressed, over-representing collagen-related and epithelial regulation pathways. Gene expression changes in E18 AVP-exposed placenta indicated potential underlying mechanisms. Deficits in behavior and forebrain development in this AVP-based preeclampsia model were distinctly different in males and females, implicating different neurobiological bases.
Conflict of interest statement
M.K.S., D.A.S., and J.L.G. hold patents related to AVP for the prediction and treatment of preeclampsia: US 293 #9,937,182 (April 10, 2018), EU #2,954,324 (July 31, 2019), and PCT/US2018/027152. The authors declare that they have no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

