Comparison of DNA repair and radiosensitivity of different blood cell populations
- PMID: 33510180
- PMCID: PMC7843614
- DOI: 10.1038/s41598-021-81058-1
Comparison of DNA repair and radiosensitivity of different blood cell populations
Abstract
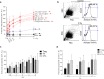
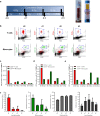
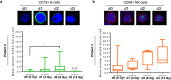
Despite the frequent use of ionising radiation (IR) in therapy and diagnostics and the unavoidable exposure to external radiation sources, our knowledge regarding the radiosensitivity of human blood cell populations is limited and published data, obtained under different experimental conditions, are heterogeneous. To compare the radiosensitivity of different hematopoietic cell populations, we set out to determine the responses of cells obtained from peripheral blood of healthy volunteers under identical conditions (resting, non-stimulated cells). First, we measured the radiation response of T cells (Treg, Th, CTL), B cells, NK cells, CD34+ progenitor cells and monocytes obtained from peripheral blood and monocyte-derived macrophages (Mph) and immature dendritic cells (iDC) ex vivo and show that T and B cells are highly sensitive, starting to undergo apoptosis following IR with a dose as low as 0.125 Gy. Importantly, there was no clear threshold dose and cell death/apoptosis increased up to a saturation level with a dose of 2 Gy. The sensitivity decreased in the order of T cells > NK and B cells > monocytes > macrophages and iDC. The data confirm a previous report that Mph and iDC are radiation-resistant compared to their progenitor monocytes. Although non-stimulated T and B cells were highly radiation-sensitive compared to monocytes and macrophages, they were competent in the repair of DNA double-strand breaks, as shown by a decline in γH2AX foci in the post-exposure period. CD34+ cells obtained from peripheral blood also showed γH2AX decline post-exposure, indicating they are repair competent. Granulocytes (CD15+) did not display any γH2AX staining following IR. Although peripheral blood lymphocytes, the main fraction are T cells, were significantly more radiation-sensitive than monocytes, they displayed the expression of the repair proteins XRCC1, ligase III and PARP-1, which were nearly non-expressed in monocytes. To assess whether monocytes are depleted in vivo following IR, we measured the amount of T cells and monocytes in cancer patients who received total-body radiation (TBR, 6 × 2 Gy). We observed that the number of T cells in the peripheral blood significantly declined already after the first day of TBR and remained at a low level, which was accompanied by an increase in the number of γH2AX foci in the surviving CD3+ T cell fraction. In contrast, the number of monocytes did not decline extensively, reflecting their radiation resistance compared to T cells. Monocytes also showed an accumulation of γH2AX foci in vivo, but the levels were significantly lower than in T cells. CD56+ NK cells displayed a response similar to T cells. The data support the notion that unstimulated T cell subfractions are nearly equally radiation sensitive. There are, however, remarkable differences in the radiation sensitivity between the lymphoid and the myeloid lineage, with lymphoid cells being significantly more sensitive than cells of the myeloid lineage. In the myeloid lineage, macrophages and iDCs were the most radio-resistant cell types.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

