Clinical pattern of failure after a durable response to immune check inhibitors in non-small cell lung cancer patients
- PMID: 33510255
- PMCID: PMC7844257
- DOI: 10.1038/s41598-021-81666-x
Clinical pattern of failure after a durable response to immune check inhibitors in non-small cell lung cancer patients
Abstract
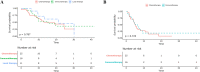
Although immune checkpoint inhibitors (ICIs) can induce durable responses in non-small-cell lung cancer (NSCLC) patients, a significant proportion of responders still experience progressive disease after a period of response. Limited data are available on the clinical patterns of acquired resistance (AR) to ICIs. Clinical and radiologic data from 125 NSCLC patients treated with anti-PD-1 or PD-L1 antibodies between 2011 and 2018 at two tertiary academic institutions were retrospectively reviewed. Overall, 63 (50.4%) patients experienced AR after ICI treatment in a median of 10.7 months. Among the 13 patients with a partial response with ICI, 12 (32.4%) had only lymph node progression. Most patients (n = 52, 82.5%) had one or two sites with progression (oligo-progression). The median overall survival (OS) after progression was significantly longer in the extrathoracic group than in the thoracic and liver progression groups (30.2 months [95% confidence interval (CI), 13.4 to not reached (NR)], 11.7 months [95% CI, 9.5-21.1], and 5.4 months [95% CI, 2.6-NR], respectively, P < 0.001). Patients with oligo-progression had significantly longer OS after AR than did the multi-progression patients (18.9 months [95% CI, 10.6-NR] vs. 8.8 months [95% CI, 5.7-NR], P = 0.04). No significant difference in progression-free survival was observed between the subsequent chemotherapy and the ICI after AR groups (P = 0.723). Patients with AR after ICI treatment had a unique progression pattern with oligo-progression and high rates of progression only in the lymph nodes. Local treatment and/or continuation of ICIs beyond AR might be an effective option.
Conflict of interest statement
The authors declare no competing interests.
Figures


Comment in
-
[Relevance of radiotherapy in the context of increasingly effective systemic therapies for metastatic NSCLC-evidence from a recurrence analysis after immune checkpoint inhibitors].Strahlenther Onkol. 2021 Dec;197(12):1151-1153. doi: 10.1007/s00066-021-01864-4. Epub 2021 Oct 26. Strahlenther Onkol. 2021. PMID: 34704118 Free PMC article. German. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

