SARS-CoV-2 infection elicits a rapid neutralizing antibody response that correlates with disease severity
- PMID: 33510275
- PMCID: PMC7843981
- DOI: 10.1038/s41598-021-81862-9
SARS-CoV-2 infection elicits a rapid neutralizing antibody response that correlates with disease severity
Abstract
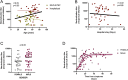
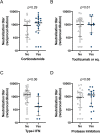
The protective effect of neutralizing antibodies in SARS-CoV-2 infected individuals is not yet well defined. To address this issue, we have analyzed the kinetics of neutralizing antibody responses and their association with disease severity. Between March and May 2020, the prospective KING study enrolled 72 COVID-19+ participants grouped according to disease severity. SARS-CoV-2 infection was diagnosed by serological and virological tests. Plasma neutralizing responses were assessed against replicative virus and pseudoviral particles. Multiple regression and non-parametric tests were used to analyze dependence of parameters. The magnitude of neutralizing titers significantly increased with disease severity. Hospitalized individuals developed higher titers compared to mild-symptomatic and asymptomatic individuals, which together showed titers below the detection limit in 50% of cases. Longitudinal analysis confirmed the strong differences in neutralizing titers between non-hospitalized and hospitalized participants and showed rapid kinetics of appearance of neutralizing antibodies (50% and 80% of maximal activity reached after 11 and 17 days after symptoms onset, respectively) in hospitalized patients. No significant impact of age, gender or treatment on the neutralizing titers was observed in this limited cohort. These data identify a clear association of humoral immunity with disease severity and point to immune mechanisms other than antibodies as relevant players in COVID-19 protection.
Conflict of interest statement
Outside the submitted work JB and JC are founders and shareholders of AlbaJuna Therapeutics, S.L. BC is founder and shareholder of AlbaJuna Therapeutics, S.L and AELIX Therapeutics, S.L. The other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

