Natural products in drug discovery: advances and opportunities
- PMID: 33510482
- PMCID: PMC7841765
- DOI: 10.1038/s41573-020-00114-z
Natural products in drug discovery: advances and opportunities
Abstract
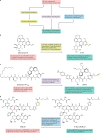
Natural products and their structural analogues have historically made a major contribution to pharmacotherapy, especially for cancer and infectious diseases. Nevertheless, natural products also present challenges for drug discovery, such as technical barriers to screening, isolation, characterization and optimization, which contributed to a decline in their pursuit by the pharmaceutical industry from the 1990s onwards. In recent years, several technological and scientific developments - including improved analytical tools, genome mining and engineering strategies, and microbial culturing advances - are addressing such challenges and opening up new opportunities. Consequently, interest in natural products as drug leads is being revitalized, particularly for tackling antimicrobial resistance. Here, we summarize recent technological developments that are enabling natural product-based drug discovery, highlight selected applications and discuss key opportunities.
Conflict of interest statement
A.G.A. is executive administrator of the International Natural Product Sciences Taskforce (INPST) and Digital Health and Patient Safety Platform (DHPSP). M. Banach has served on the speakers’ bureau of Abbott/Mylan, Abbott Vascular, Actavis, Akcea, Amgen, Biofarm, KRKA, MSD, Novo-Nordisk, Novartis, Sanofi-Aventis, Servier and Valeant, has served as a consultant to Abbott Vascular, Akcea, Amgen, Daichii Sankyo, Esperion, Freia Pharmaceuticals, Lilly, MSD, Novartis, Polfarmex, Resverlogix, Sanofi-Aventis, and has received grants from Amgen, Mylan, Sanofi and Valeant. R.B. collaborates with Bayer Consumer Health and Dr Willmar Schwabe GmbH & Co. KG, and is scientific advisory committee member of PuraPharm International (HK) Limited and ISURA. G.K.B. is a board member of Bionorica SE. M. Daglia has received consultancy honoraria from Pfizer Italia and Mylan for training courses for chemists, and is a member of the INPST board of directors. A.T.D.-K. is a member of the Scientific and Medical Advisory Board of Evgen Pharma plc. I.E.O. is Dean of Faculty of Pharmacy, Gazi University, Ankara, Turkey, member of the Traditional Chinese Medicine Experts Group in European Pharmacopeia, and principal member of Turkish Academy of Sciences (TUBA). B.L.F. is a member of the INPST Board of Directors and has received research funding from Dr Willmar Schwabe GmbH & Co. KG. K.M.G. has received reimbursement for speaking at conferences sponsored by companies selling nutritional products and is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone. C.W.G. is chairman of the scientific advisory board of Cyxone AB, SE. M.H.’s research group has received charitable donations from Dr Willmar Schwabe GmbH & Co. KG and recently completed a research project sponsored by Pukka Herbs, UK. A.L. is a member of the board of directors of Kaisa Health. M.J.S.M. is president of Kaiviti Consulting and consults for Gnosis by LeSaffre. F.N. is cofounder and shareholder of OncoNox and Aura Biopharm. G.P. is on the board of Neurotez and Neurotrope. M.R. serves as an adviser for the Nestlé Institute of Health Sciences. G.L.R. is a member of the board of directors of INPST. N.T.T. is Founder and CEO of NTZ Lab Ltd and advisory board member of INPST. M.W. collaborates with Finzelberg GmbH and Schwabe GmbH. J.L.W. collaborates with Nestlé and Firmenich. M.A.P. is CEO and owner of Bionorica SE. J.H. is an employee of and holds shares in UCB Pharma Ltd. M.M. is Founder and Chairman of Sami–Sabinsa Group of Companies. D.S.B. is an employee of Janssen R&D. M. Bodkin is an employee of Evotec (UK) Ltd.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

