Human Adaptations to Multiday Saturation on NASA NEEMO
- PMID: 33510647
- PMCID: PMC7835980
- DOI: 10.3389/fphys.2020.610000
Human Adaptations to Multiday Saturation on NASA NEEMO
Abstract
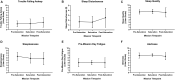
Human adaptation to extreme environments has been explored for over a century to understand human psychology, integrated physiology, comparative pathologies, and exploratory potential. It has been demonstrated that these environments can provide multiple external stimuli and stressors, which are sufficient to disrupt internal homeostasis and induce adaptation processes. Multiday hyperbaric and/or saturated (HBS) environments represent the most understudied of environmental extremes due to inherent experimental, analytical, technical, temporal, and safety limitations. National Aeronautic Space Agency (NASA) Extreme Environment Mission Operation (NEEMO) is a space-flight analog mission conducted within Florida International University's Aquarius Undersea Research Laboratory (AURL), the only existing operational and habitable undersea saturated environment. To investigate human objective and subjective adaptations to multiday HBS, we evaluated aquanauts living at saturation for 9-10 days via NASA NEEMO 22 and 23, across psychologic, cardiac, respiratory, autonomic, thermic, hemodynamic, sleep, and body composition parameters. We found that aquanauts exposed to saturation over 9-10 days experienced intrapersonal physical and mental burden, sustained good mood and work satisfaction, decreased heart and respiratory rates, increased parasympathetic and reduced sympathetic modulation, lower cerebral blood flow velocity, intact cerebral autoregulation and maintenance of baroreflex functionality, as well as losses in systemic bodyweight and adipose tissue. Together, these findings illustrate novel insights into human adaptation across multiple body systems in response to multiday hyperbaric saturation.
Keywords: NASA; adaptation; extreme environment; hyperbaric (underwater); saturation.
Copyright © 2021 Koutnik, Favre, Noboa, Sanchez-Gonzalez, Moss, Goubran, Ari, Poff, Rogers, DeBlasi, Samy, Moussa, Serrador and D’Agostino.
Conflict of interest statement
The authors declare that this study received funding from Ketone Technologies LLC. Ketone Technologies LLC is an entity which supports research on extreme environments for which CA and DD serve as CEO and CSO, respectively. CA and DD received no personal compensation for these studies and were involved in project development, logistical implementation, and final manuscript development. These interests have been reviewed and managed by NASA in accordance with its Institutional and Individual Conflict of Interest policies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Adamiec L. (1977). Effect of hyperbaric oxygen therapy on some basic vital functions. Acta Physiol. Pol. 28 215–224. - PubMed
-
- Ainslie P. N., Ogoh S., Burgess K., Celi L., McGrattan K., Peebles K., et al. (2008). Differential effects of acute hypoxia and high altitude on cerebral blood flow velocity and dynamic cerebral autoregulation: alterations with hyperoxia. J. Appl. Physiol. 104 490–498. 10.1152/japplphysiol.00778.2007 - DOI - PubMed
-
- Anderson D. G., Herrmann H. G. (1971). Technical Report - Seafloor Foundations: Analysis of Case Histories. Port Hueneme, CA: Naval Civil Engineering Laboratory.
-
- Ari C. S., D’Agostino D. P., Bharwani S., Rehsi A., Moss S., Schmer-Galunder S., et al. (2020c). Changes in Motor Function in Response to Living in an Extreme Underwater Saturation Environment under High Stress and Increased Workload. FASEB J. 34:9603 10.1096/fasebj.2020.34.s1.09603 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

