Omalizumab treatment and outcomes in Chinese patients with chronic spontaneous urticaria, chronic inducible urticaria, or both
- PMID: 33510832
- PMCID: PMC7804987
- DOI: 10.1016/j.waojou.2020.100501
Omalizumab treatment and outcomes in Chinese patients with chronic spontaneous urticaria, chronic inducible urticaria, or both
Abstract
Background: Chronic urticaria (CU) is a common skin disorder, which can be further divided into chronic spontaneous urticaria (CSU) and chronic inducible urticaria (CIndU). Omalizumab is effective and safe for difficult-to-treat CSU based on clinical trials. However, there are limited data comparing the therapeutic effect of omalizumab for patients with CSU, CIndU, and CSU plus CIndU. Meanwhile, there is still no reliable predictor for treatment response or relapse. Our study was conducted to collect real-world clinical data on omalizumab treatment in patients with CSU, CIndU, and both.
Methods: This was an observational, retrospective chart review of patients with CU initiating omalizumab treatment between February 2018 and May 2020 (maximum 28 months follow-up).
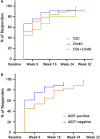
Results: A total of 138 patients were included, 87 with CSU alone, 33 with different forms of CIndU, and 18 with both. A total of 87.0% (n = 120/138) of the CU patients responded to omalizumab therapy, among which 65.2% (n = 90/138) of the patients showed complete response and 21.7% (n = 30/138) of the patients showed partial response. The therapeutic effect and speed of onset of effect for omalizumab were comparable among patients with CSU, CIndU, or both. Autologous serum skin test (ASST)-positive patients were more likely to show a slow response to omalizumab therapy ( P = 0.043). Non-responders had lower baseline total IgE levels (35.0 vs 121.5 kU/L, P < 0.001). The proportion of patients with low total IgE levels in non-responders was significantly higher than that of responders (61.1% vs. 14.5%, P < 0.001). Also, more non-responder patients had elevated thyroid autoantibodies than responders (50.0% vs. 23.0%, P = 0.041). The median ratio of serum IgG-anti-TPO to serum total IgE in non-responders was significantly higher compared with responders (1.22 vs. 0.09, P < 0.001). Non-responders also had shorter treatment periods (4.5 vs 6.0 months, P = 0.035) compared with responders. Two of 3 patients (67.4%, n = 29/43) experienced relapse after ceasing omalizumab therapy. These patients had longer disease durations (52.0 vs. 15.0 months, P = 0.007) and higher baseline total IgE levels (179.9 vs. 72.5 kU/L, P = 0.020) than patients who did not relapse. We reinitiated omalizumab treatment for 10 relapsed patients, all of them reported a rapid response after the first injection within the first 4 weeks of retreatment.
Conclusion: Omalizumab is highly effective in patients with difficult-to-treat CSU, CIndU, or both. Responders tend to have unique immunological features and longer treatment periods. Patients with higher baseline total IgE levels and longer disease durations are more likely to experience rapid relapse after discontinuation of omalizumab.
Keywords: Chronic urticaria; Dermatology life quality index; Omalizumab; Urticaria control test.
© 2020 The Author(s).
Conflict of interest statement
Yudi Chen: none; Miao Yu: none; Xiaojie Huang: none; Ping Tu: none; Peikun Shi: none; Marcus Maurer: Speaker and/or advisor and recipient of institutional research funding from 10.13039/100004328Genentech and 10.13039/100004336Novartis; Zuotao Zhao: research support from 10.13039/100004336Novartis outside the submitted work.
Figures


Similar articles
-
Analysis of the Efficacy and Recurrence of Omalizumab Use in the Treatment of Chronic Spontaneous Urticaria and Chronic Inducible Urticaria.Int Arch Allergy Immunol. 2023;184(7):643-655. doi: 10.1159/000529250. Epub 2023 Mar 30. Int Arch Allergy Immunol. 2023. PMID: 36996780
-
Anti-TPO IgG/Total IgE Ratio: Biomarker for Omalizumab Response Prediction in Chronic Spontaneous Urticaria.Int Arch Allergy Immunol. 2023;184(9):866-869. doi: 10.1159/000532021. Epub 2023 Aug 9. Int Arch Allergy Immunol. 2023. PMID: 37557083
-
Management of chronic inducible urticaria according to the guidelines: A prospective controlled study.J Dermatol Sci. 2017 Jul;87(1):60-69. doi: 10.1016/j.jdermsci.2017.02.283. Epub 2017 Mar 1. J Dermatol Sci. 2017. PMID: 28314658
-
Biomarkers of Autoimmune Chronic Spontaneous Urticaria.Curr Allergy Asthma Rep. 2023 Dec;23(12):655-664. doi: 10.1007/s11882-023-01117-7. Epub 2023 Dec 8. Curr Allergy Asthma Rep. 2023. PMID: 38064133 Review.
-
Expert consensus on the use of omalizumab in chronic urticaria in China.World Allergy Organ J. 2021 Dec 5;14(11):100610. doi: 10.1016/j.waojou.2021.100610. eCollection 2021 Nov. World Allergy Organ J. 2021. PMID: 34934470 Free PMC article. Review.
Cited by
-
Case report: Severe chronic spontaneous urticaria successfully treated with omalizumab and dupilumab.Allergol Select. 2023 Jan 3;7:17-19. doi: 10.5414/ALX02382E. eCollection 2023. Allergol Select. 2023. PMID: 36644012 Free PMC article.
-
Targeted therapy for immune mediated skin diseases. What should a dermatologist know?An Bras Dermatol. 2024 Jul-Aug;99(4):546-567. doi: 10.1016/j.abd.2023.10.002. Epub 2024 Mar 22. An Bras Dermatol. 2024. PMID: 38521706 Free PMC article.
-
Factors Influencing Relapse After Omalizumab in Chronic Urticaria. Does the Method of Discontinuation Influence Relapse?Dermatol Pract Concept. 2025 Jul 31;15(3):5196. doi: 10.5826/dpc.1503a5196. Dermatol Pract Concept. 2025. PMID: 40790459 Free PMC article.
-
[Anti-IgE-directed treatment of urticaria in a dermatological practice].Dermatologie (Heidelb). 2022 Oct;73(10):788-794. doi: 10.1007/s00105-022-05023-3. Epub 2022 Jun 22. Dermatologie (Heidelb). 2022. PMID: 35925212 Free PMC article. German.
-
Biomarkers for Monitoring Treatment Response of Omalizumab in Patients with Chronic Urticaria.Int J Mol Sci. 2023 Jul 11;24(14):11328. doi: 10.3390/ijms241411328. Int J Mol Sci. 2023. PMID: 37511088 Free PMC article. Review.
References
-
- Fricke J., Ávila G., Keller T. Prevalence of chronic urticaria in children and adults across the globe: systematic review with meta-analysis. Allergy. 2020;75(2):423–432. - PubMed
-
- Zuberbier T., Aberer W., Asero R. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73(7):1393–1414. - PubMed
-
- Maurer M., Weller K., Bindslev-Jensen C. Unmet clinical needs in chronic spontaneous urticaria. A GA2LEN task force report. Allergy. 2011;66(3):317–330. - PubMed
-
- Kolkhir P., Church M.K., Weller K., Metz M., Schmetzer O., Maurer M. Autoimmune chronic spontaneous urticaria: what we know and what we do not know. J Allergy Clin Immunol. 2017;139(6):1772–1781. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

