Abnormal Nutritive Sucking as an Indicator of Neonatal Brain Injury
- PMID: 33511093
- PMCID: PMC7835320
- DOI: 10.3389/fped.2020.599633
Abnormal Nutritive Sucking as an Indicator of Neonatal Brain Injury
Abstract
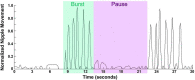
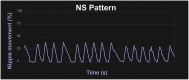
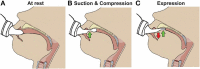
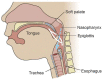
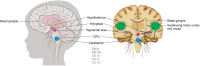
A term neonate is born with the ability to suck; this neuronal network is already formed and functional by 28 weeks gestational age and continues to evolve into adulthood. Because of the necessity of acquiring nutrition, the complexity of the neuronal network needed to suck, and neuroplasticity in infancy, the skill of sucking has the unique ability to give insight into areas of the brain that may be damaged either during or before birth. Interpretation of the behaviors during sucking shows promise in guiding therapies and how to potentially repair the damage early in life, when neuroplasticity is high. Sucking requires coordinated suck-swallow-breathe actions and is classified into two basic types, nutritive and non-nutritive. Each type of suck has particular characteristics that can be measured and used to learn about the infant's neuronal circuitry. Basic sucking and swallowing are present in embryos and further develop to incorporate breathing ex utero. Due to the rhythmic nature of the suck-swallow-breathe process, these motor functions are controlled by central pattern generators. The coordination of swallowing, breathing, and sucking is an enormously complex sensorimotor process. Because of this complexity, brain injury before birth can have an effect on these sucking patterns. Clinical assessments allow evaluators to score the oral-motor pattern, however, they remain ultimately subjective. Thus, clinicians are in need of objective measures to identify the specific area of deficit in the sucking pattern of each infant to tailor therapies to their specific needs. Therapeutic approaches involve pacifiers, cheek/chin support, tactile, oral kinesthetic, auditory, vestibular, and/or visual sensorimotor inputs. These therapies are performed to train the infant to suck appropriately using these subjective assessments along with the experience of the therapist (usually a speech therapist), but newer, more objective measures are coming along. Recent studies have correlated pathological sucking patterns with neuroimaging data to get a map of the affected brain regions to better inform therapies. The purpose of this review is to provide a broad scope synopsis of the research field of infant nutritive and non-nutritive feeding, their underlying neurophysiology, and relationship of abnormal activity with brain injury in preterm and term infants.
Keywords: brain injury; neuroimaging; non-nutritive; nutritive; sucking.
Copyright © 2021 Shandley, Capilouto, Tamilia, Riley, Johnson and Papadelis.
Conflict of interest statement
GC is employed by the company NFANT Labs, LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

