Epigenetic Regulation of Adipogenesis in Development of Metabolic Syndrome
- PMID: 33511131
- PMCID: PMC7835429
- DOI: 10.3389/fcell.2020.619888
Epigenetic Regulation of Adipogenesis in Development of Metabolic Syndrome
Abstract
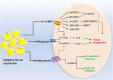
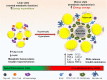
Obesity is one of the biggest public health concerns identified by an increase in adipose tissue mass as a result of adipocyte hypertrophy and hyperplasia. Pertaining to the importance of adipose tissue in various biological processes, any alteration in its function results in impaired metabolic health. In this review, we discuss how adipose tissue maintains the metabolic health through secretion of various adipokines and inflammatory mediators and how its dysfunction leads to the development of severe metabolic disorders and influences cancer progression. Impairment in the adipocyte function occurs due to individuals' genetics and/or environmental factor(s) that largely affect the epigenetic profile leading to altered gene expression and onset of obesity in adults. Moreover, several crucial aspects of adipose biology, including the regulation of different transcription factors, are controlled by epigenetic events. Therefore, understanding the intricacies of adipogenesis is crucial for recognizing its relevance in underlying disease conditions and identifying the therapeutic interventions for obesity and metabolic syndrome.
Keywords: adipogenesis; insulin resistance; metabolic syndrome; obesity; transgenerational inheritance.
Copyright © 2021 Pant, Firmal, Shah, Alam and Chattopadhyay.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

