Bearing My Heart: The Role of Extracellular Matrix on Cardiac Development, Homeostasis, and Injury Response
- PMID: 33511134
- PMCID: PMC7835513
- DOI: 10.3389/fcell.2020.621644
Bearing My Heart: The Role of Extracellular Matrix on Cardiac Development, Homeostasis, and Injury Response
Abstract
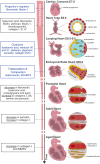
The extracellular matrix (ECM) is an essential component of the heart that imparts fundamental cellular processes during organ development and homeostasis. Most cardiovascular diseases involve severe remodeling of the ECM, culminating in the formation of fibrotic tissue that is deleterious to organ function. Treatment schemes effective at managing fibrosis and promoting physiological ECM repair are not yet in reach. Of note, the composition of the cardiac ECM changes significantly in a short period after birth, concurrent with the loss of the regenerative capacity of the heart. This highlights the importance of understanding ECM composition and function headed for the development of more efficient therapies. In this review, we explore the impact of ECM alterations, throughout heart ontogeny and disease, on cardiac cells and debate available approaches to deeper insights on cell-ECM interactions, toward the design of new regenerative therapies.
Keywords: cardiac ontogeny; cardiovascular diseases; decellularization; extracellular matrix; fibrosis; heart; regeneration.
Copyright © 2021 Silva, Pereira, Fonseca, Pinto-do-Ó and Nascimento.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Aggeler J. (1988). Three-dimensional organization of the extracellular matrix secreted by cultured rat smooth muscle cells. Vitro Cell Dev. Biol. 24 633–638. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

