Down-Regulation of Colonic ACE2 Expression in Patients With Inflammatory Bowel Disease Responding to Anti-TNF Therapy: Implications for COVID-19
- PMID: 33511147
- PMCID: PMC7835139
- DOI: 10.3389/fmed.2020.613475
Down-Regulation of Colonic ACE2 Expression in Patients With Inflammatory Bowel Disease Responding to Anti-TNF Therapy: Implications for COVID-19
Abstract
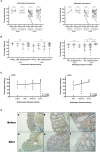
Background and Aims: Angiotensin-converting enzyme II (ACE2) is the key molecule for understanding the pathophysiology of COVID-19. The risk of COVID-19 and impact of immunosuppressive treatment on disease course in patients with inflammatory bowel disease (IBD) remain controversial. We aimed to determine the change of intestinal ACE2 expression before and after biologics treatment including anti-tumor necrosis factor α (anti-TNFα), anti-integrin, and anti-interleukin (IL)12/23 in IBD patients. Methods: We analyzed the ACE2 expression through the public database of paired intestinal biopsies from IBD patients before and after biologic therapy. Change of ACE2 RNA and protein expression were validated in two independent cohorts (Birmingham cohort and Guangzhou cohort). The correlation between ACE2 expression and disease activity was also analyzed. Results: Mining information from the GEO database showed that compared with healthy control, intestinal ACE2 expression was downregulated in ileum of CD patients, while upregulated in colon of both CD and UC patients. Colonic ACE2 RNA expression was decreased significantly in patients responding to anti-TNFα but not anti-integrin and anti-IL12/23, which was validated in the Birmingham cohort. Using the Guangzhou cohort including 53 patients matched by pre- and post-anti-TNFα therapy, colonic ACE2 protein expression was significantly downregulated after anti-TNFα treatment in responders (P < 0.001) rather than non-responders. Colonic ACE2 expression was significantly higher in patients with severe histologically active disease compared with those with moderate (P < 0.0001) and mild (P = 0.0002) histologically active disease. Conclusion: Intestinal inflammation influences the expression of intestinal ACE2 in IBD patients, with different alterations in the ileum and colon. Colonic ACE2 expression was downregulated after anti-TNFα therapy in IBD patients responding to treatment. This might provide new clues regarding the risk of SARS-CoV-2 infection and the potential benefit of sustaining anti-TNFα treatment in patients with IBD.
Keywords: ACE2; COVID-19; anti-TNFα; inflammatory bowel disease; intestine.
Copyright © 2021 Li, Qiu, Jeffery, Liu, Feng, He, Tan, Ye, Lin, Ghosh, Iacucci, Chen and Mao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, et al. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. (2020) 69:1010–8. 10.1136/gutjnl-2020-320953 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

