Poststudy Point-of-Care Oral Fluid Testing in Human Immunodeficiency Virus-1 Vaccinees
- PMID: 33511233
- PMCID: PMC7813203
- DOI: 10.1093/ofid/ofaa606
Poststudy Point-of-Care Oral Fluid Testing in Human Immunodeficiency Virus-1 Vaccinees
Abstract
Background: Experimental human immunodeficiency virus (HIV)-1 vaccines frequently elicit antibodies against HIV-1 that may react with commonly used HIV diagnostic tests, a phenomenon known as vaccine-induced seropositivity/seroreactivity (VISP/VISR). We sought to determine, under clinic conditions, whether a patient-controlled HIV test, OraQuick ADVANCE Rapid HIV-1/2 Antibody Test, detected HIV-1 vaccine-induced antibodies.
Methods: Plasma assessment of HIV-1 cross-reactivity was examined in end-of-study samples from 57 healthy, HIV-uninfected participants who received a candidate vaccine that has entered Phase 2B and 3 testing. We also screened 120 healthy, HIV-uninfected, unblinded HIV-1 vaccine participants with VISP/VISR for an assessment using saliva. These participants came from 21 different parent vaccine protocols representing 17 different vaccine regimens, all of which contained an HIV-1 envelope immunogen. OraQuick ADVANCE was compared with results from concurrent blood samples using a series of commercial HIV screening immunoassays.
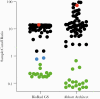
Results: Fifty-seven unique participant plasma samples were assayed in vitro, and only 1 (1.8%) was reactive by OraQuick ADVANCE. None of the 120 clinic participants (0%; 95% confidence interval, 0% to 3.7%) tested positive by OraQuick ADVANCE, and all were confirmed to be uninfected by HIV-1 viral ribonucleic acid testing. One hundred eighteen of the 120 (98.3%) participants had a reactive HIV test for VISP/VISR: 77 (64%) had at least 1 reactive fourth-generation HIV-1 diagnostic test (P < .0001 vs no reactive OraQuick ADVANCE results), and 41 (34%) only had a reactive test by the less specific third-generation Abbott Prism assay.
Conclusions: These data suggest that this widely available patient-controlled test has limited reactivity to HIV-1 antibodies elicited by these candidate HIV-1 vaccines.
Keywords: HIV diagnostics; HIV vaccine; immunogenicity; vaccine safety.
© The Author(s) 2020. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures
References
-
- Pitisuttithum P, Marovich MA. Prophylactic HIV vaccine: vaccine regimens in clinical trials and potential challenges. Expert Rev Vaccines 2020; 19:133–42. - PubMed
-
- Russell ND, Marovich MA. Pox-protein public private partnership program and upcoming HIV vaccine efficacy trials. Curr Opin HIV AIDS 2016; 11:614–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

