Stem Cell Therapy Potency in Personalizing Severe COVID-19 Treatment
- PMID: 33511518
- PMCID: PMC7842180
- DOI: 10.1007/s12015-020-10110-w
Stem Cell Therapy Potency in Personalizing Severe COVID-19 Treatment
Abstract
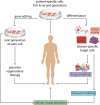
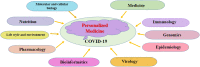
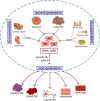
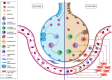
Currently, there are no specific and efficient vaccines or drugs for COVID-19, particularly in severe cases. A wide range of variations in the clinical symptoms of different patients attributed to genomic differences. Therefore, personalized treatments seem to play a critical role in improving these symptoms and even similar conditions. Prompted by the uncertainties in the area of COVID-19 therapies, we reviewed the published papers and concepts to gather and provide useful information to clinicians and researchers interested in personalized medicine and cell-based therapy. One novel aspect of this study focuses on the potential application of personalized medicine in treating severe cases of COVID-19. However, it is theoretical, as any real-world examples of the use of genuinely personalized medicine have not existed yet. Nevertheless, we know that stem cells, especially MSCs, have immune-modulatory effects and can be stored for future personalized medicine applications. This theory has been conjugated with some evidence that we review in the present study. Besides, we discuss the importance of personalized medicine and its possible aspects in COVID-19 treatment, then review the cell-based therapy studies for COVID-19 with a particular focus on stem cell-based therapies as a primary personalized tool medicine. However, the idea of cell-based therapy has not been accepted by several scientific communities due to some concerns of lack of satisfactory clinical studies; still, the MSCs and their clinical outcomes have been revealed the safety and potency of this therapeutic approach in several diseases, especially in the immune-mediated inflammatory diseases and some incurable diseases. Promising outcomes have resulted in that clinical studies are going to continue.
Keywords: COVID-19; Cell-based therapy; Personalized medicine; Stem cell.
Figures

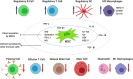
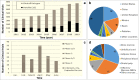
References
-
- Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak- a n update on the status. Military Medical Research. BioMed Central Ltd. 2020;7:11. doi: 10.1186/s40779-020-00240-0. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

