Tocilizumab in COVID-19 interstitial pneumonia
- PMID: 33511686
- PMCID: PMC8013903
- DOI: 10.1111/joim.13231
Tocilizumab in COVID-19 interstitial pneumonia
Abstract
Background: Published reports on tocilizumab in COVID-19 pneumonitis show conflicting results due to weak designs or heterogeneity in critical methodological issues.
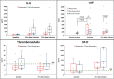
Methods: This open-label trial, structured according to Simon's optimal design, aims to identify factors predicting which patients could benefit from anti-IL6 strategies and to enhance the design of unequivocal and reliable future randomized trials. A total of 46 patients with COVID-19 pneumonia needing of oxygen therapy to maintain SO2 > 93% and with recent worsening of lung function received a single infusion of tocilizumab. Clinical and biological markers were measured to test their predictive values. Primary end point was early and sustained clinical response.
Results: Twenty-one patients fulfilled pre-defined response criteria. Lower levels of IL-6 at 24 h after tocilizumab infusion (P = 0.049) and higher baseline values of PaO2/FiO2 (P = 0.008) predicted a favourable response.
Conclusions: Objective clinical response rate overcame the pre-defined threshold of 30%. Efficacy of tocilizumab to improve respiratory function in patients selected according to our inclusion criteria warrants investigations in randomized trials.
Keywords: COVID-19; IL-6; pneumonia; severe acute respiratory syndrome; tocilizumab.
© 2020 The Association for the Publication of the Journal of Internal Medicine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

