How we treat Merkel cell carcinoma: within and beyond current guidelines
- PMID: 33511866
- PMCID: PMC7983043
- DOI: 10.2217/fon-2020-1036
How we treat Merkel cell carcinoma: within and beyond current guidelines
Abstract
Merkel cell carcinoma (MCC) is an aggressive skin cancer associated with a high risk of local recurrence and distant metastasis. Optimal care of this potentially life-threatening cancer is critical but challenging because: physicians are often unfamiliar with its management due to rarity, and MCC management remains controversial, in part because it is rapidly evolving across multiple specialties. While guidelines offer a broad overview of management, they are often not sufficient when making decisions for individual patients. Herein, we present a literature review as well as practical approaches adopted at our institutions for staging, surveillance and therapy of MCC. Each of these areas are discussed in light of how they can be appropriately customized for prevalent but challenging situations. We also provide representative examples of MCC patient scenarios and how they were managed by a multidisciplinary team to identify suitable evidence-based, individualized treatment plans.
Keywords: Merkel cell carcinoma; customized treatment; imaging; immunotherapy; multidisciplinary management; radiotherapy; surgery; surveillance.
Plain language summary
Lay abstract Merkel cell carcinoma (MCC) is a skin cancer with a high risk of recurrence and distant spread. Optimal care of this cancer is important. However, management is challenging because it is rare and its treatment is continuously evolving across multiple specialties. While treatment guidelines offer a broad overview of management, they are often not detailed enough to provide appropriate patient-specific assistance. Herein, we present a review of recent studies and our suggestions relevant to MCC staging, surveillance and treatment options. Each of these areas are discussed in light of how they can be appropriately customized for challenging situations often encountered by practitioners. We also provide representative examples of MCC patient scenarios and how they were managed by a multidisciplinary team to identify evidence-based, individualized treatment plans.
Conflict of interest statement
This research was made possible by funding from NIH/NCI R01CA162522, NIH/NCI P01CA225517, NIH/NCI Cancer Center Support Grant P30 CA015704, the MCC patient gift fund at University of Washington, and Kelsey Dickson Team Science Courage Research Team Award from the Prostate Cancer Foundation (award #15CHAS04), and a ‘Friends and Family of Nancy Haeseker’ fund’. P Nghiem has received consulting fees from EMD Serono, Pfizer, Sanofi/Regeneron, 4SC and research grant support from Bristol-Meyers Squibb; S Bhatia has received consulting fees from Genentech, EMD Serono, Bristol Myers Squibb, Sanofi Genzyme and Exicure; and research funding to his institution (University of Washington) from Oncosec, EMD Serono, Merck, BMS, NantKwest, Immune Design, Novartis, Nektar and Exicure. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures
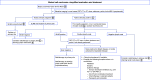
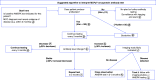
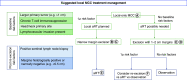
References
-
- Becker JC. Merkel cell carcinoma. Ann. Oncol. 21(Suppl. 7), vii81–vii85 (2010). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
