Therapeutic inhibition of HIF-2α reverses polycythemia and pulmonary hypertension in murine models of human diseases
- PMID: 33512384
- PMCID: PMC8109019
- DOI: 10.1182/blood.2020009138
Therapeutic inhibition of HIF-2α reverses polycythemia and pulmonary hypertension in murine models of human diseases
Abstract
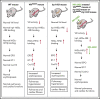
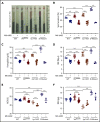
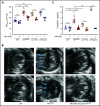
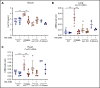
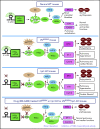
Polycythemia and pulmonary hypertension are 2 human diseases for which better therapies are needed. Upregulation of hypoxia-inducible factor-2α (HIF-2α) and its target genes, erythropoietin (EPO) and endothelin-1, causes polycythemia and pulmonary hypertension in patients with Chuvash polycythemia who are homozygous for the R200W mutation in the von Hippel Lindau (VHL) gene and in a murine mouse model of Chuvash polycythemia that bears the same homozygous VhlR200W mutation. Moreover, the aged VhlR200W mice developed pulmonary fibrosis, most likely due to the increased expression of Cxcl-12, another Hif-2α target. Patients with mutations in iron regulatory protein 1 (IRP1) also develop polycythemia, and Irp1-knockout (Irp1-KO) mice exhibit polycythemia, pulmonary hypertension, and cardiac fibrosis attributable to translational derepression of Hif-2α, and the resultant high expression of the Hif-2α targets EPO, endothelin-1, and Cxcl-12. In this study, we inactivated Hif-2α with the second-generation allosteric HIF-2α inhibitor MK-6482 in VhlR200W, Irp1-KO, and double-mutant VhlR200W;Irp1-KO mice. MK-6482 treatment decreased EPO production and reversed polycythemia in all 3 mouse models. Drug treatment also decreased right ventricular pressure and mitigated pulmonary hypertension in VhlR200W, Irp1-KO, and VhlR200W;Irp1-KO mice to near normal wild-type levels and normalized the movement of the cardiac interventricular septum in VhlR200Wmice. MK-6482 treatment reduced the increased expression of Cxcl-12, which, in association with CXCR4, mediates fibrocyte influx into the lungs, potentially causing pulmonary fibrosis. Our results suggest that oral intake of MK-6482 could represent a new approach to treatment of patients with polycythemia, pulmonary hypertension, pulmonary fibrosis, and complications caused by elevated expression of HIF-2α.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

Comment in
-
HIF-2 inhibitor, erythrocytosis, and pulmonary hypertension.Blood. 2021 May 6;137(18):2424-2425. doi: 10.1182/blood.2020010323. Blood. 2021. PMID: 33956067 No abstract available.
Similar articles
-
The von Hippel-Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice.J Clin Invest. 2010 Mar;120(3):827-39. doi: 10.1172/JCI36362. Epub 2010 Feb 8. J Clin Invest. 2010. PMID: 20197624 Free PMC article.
-
Translational repression of HIF2α expression in mice with Chuvash polycythemia reverses polycythemia.J Clin Invest. 2018 Apr 2;128(4):1317-1325. doi: 10.1172/JCI97684. Epub 2018 Feb 26. J Clin Invest. 2018. PMID: 29480820 Free PMC article.
-
Deletion of iron regulatory protein 1 causes polycythemia and pulmonary hypertension in mice through translational derepression of HIF2α.Cell Metab. 2013 Feb 5;17(2):271-81. doi: 10.1016/j.cmet.2012.12.016. Cell Metab. 2013. PMID: 23395173 Free PMC article.
-
Update on mutations in the HIF: EPO pathway and their role in erythrocytosis.Blood Rev. 2019 Sep;37:100590. doi: 10.1016/j.blre.2019.100590. Epub 2019 Jul 16. Blood Rev. 2019. PMID: 31350093 Free PMC article. Review.
-
Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis.Blood. 2009 Sep 3;114(10):2015-9. doi: 10.1182/blood-2009-05-189985. Epub 2009 Jun 3. Blood. 2009. PMID: 19494350 Review.
Cited by
-
Molecular Insights into the Oxygen-Sensing Pathway and Erythropoietin Expression Regulation in Erythropoiesis.Int J Mol Sci. 2021 Jun 30;22(13):7074. doi: 10.3390/ijms22137074. Int J Mol Sci. 2021. PMID: 34209205 Free PMC article. Review.
-
VHL ameliorates arecoline-induced oral submucosal fibrosis by promoting HDAC6 ubiquitination and blocking NF-κB pathway.Sci Rep. 2025 Mar 4;15(1):7563. doi: 10.1038/s41598-025-91207-5. Sci Rep. 2025. PMID: 40038412 Free PMC article.
-
Belzutifan-Associated Hypoxia: A Review of the Novel Therapeutic, Proposed Mechanisms of Hypoxia, and Management Recommendations.Int J Mol Sci. 2025 Jul 23;26(15):7094. doi: 10.3390/ijms26157094. Int J Mol Sci. 2025. PMID: 40806229 Free PMC article. Review.
-
Transcription factors in the pathogenesis of pulmonary arterial hypertension-Current knowledge and therapeutic potential.Front Cardiovasc Med. 2023 Jan 6;9:1036096. doi: 10.3389/fcvm.2022.1036096. eCollection 2022. Front Cardiovasc Med. 2023. PMID: 36684555 Free PMC article. Review.
-
Attention-based deep learning for accurate cell image analysis.Sci Rep. 2025 Jan 8;15(1):1265. doi: 10.1038/s41598-025-85608-9. Sci Rep. 2025. PMID: 39779905 Free PMC article.
References
-
- Bento C. Genetic basis of congenital erythrocytosis. Int J Lab Hematol. 2018;40(suppl 1):62-67. - PubMed
-
- Oliveira JL, Coon LM, Frederick LA, et al. . Genotype-Phenotype Correlation of Hereditary Erythrocytosis Mutations, a single center experience. Am J Hematol. 2018;93(8):1029-1041. - PubMed
-
- Semenza GL. The Genomics and Genetics of Oxygen Homeostasis. Annu Rev Genomics Hum Genet. 2020;21(1):183-204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

