T-cell activation profiles distinguish hemophagocytic lymphohistiocytosis and early sepsis
- PMID: 33512385
- PMCID: PMC8085480
- DOI: 10.1182/blood.2020009499
T-cell activation profiles distinguish hemophagocytic lymphohistiocytosis and early sepsis
Abstract
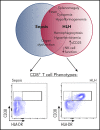
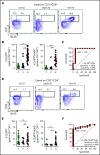
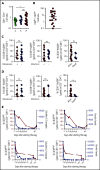
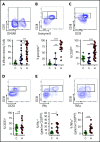
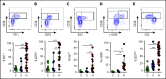
Hemophagocytic lymphohistiocytosis (HLH) is a fatal disorder of immune hyperactivation that has been described as a cytokine storm. Sepsis due to known or suspected infection has also been viewed as a cytokine storm. Although clinical similarities between these syndromes suggest similar immunopathology and may create diagnostic uncertainty, distinguishing them is critical as treatments are widely divergent. We examined T-cell profiles from children with either HLH or sepsis and found that HLH is characterized by acute T-cell activation, in clear contrast to sepsis. Activated T cells in patients with HLH were characterized as CD38high/HLA-DR+ effector cells, with activation of CD8+ T cells being most pronounced. Activated T cells were type 1 polarized, proliferative, and displayed evidence of recent and persistent activation. Circulating activated T cells appeared to be broadly characteristic of HLH, as they were seen in children with and without genetic lesions or identifiable infections and resolved with conventional treatment of HLH. Furthermore, we observed even greater activation and type 1 polarization in tissue-infiltrating T cells, described here for the first time in a series of patients with HLH. Finally, we observed that a threshold of >7% CD38high/HLA-DR+ cells among CD8+ T cells had strong positive and negative predictive value for distinguishing HLH from early sepsis or healthy controls. We conclude that the cytokine storm of HLH is marked by distinctive T-cell activation whereas early sepsis is not, and that these 2 syndromes can be readily distinguished by T-cell phenotypes.
© 2021 by the American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: M.B.J., M.L.H., and C.E.A. have served as consultants for Sobi. M.L.H. serves on a data safety monitoring committee for Novimmune. The remaining authors declare no competing financial interests.
Figures

Comment in
-
HLH or sepsis: the truth is in the T cells.Blood. 2021 Apr 29;137(17):2279-2280. doi: 10.1182/blood.2020010236. Blood. 2021. PMID: 33914076 No abstract available.
References
-
- Sriskandan S, Altmann DM. The immunology of sepsis. J Pathol. 2008;214(2):211-223. - PubMed
-
- Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11(8):532-543. - PubMed
-
- Ferrara JL, Abhyankar S, Gilliland DG. Cytokine storm of graft-versus-host disease: a critical effector role for interleukin-1. Transplant Proc. 1993;25(1 pt 2):1216-1217. - PubMed
-
- Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355(10):1018-1028. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

