Long-term outcomes with emicizumab prophylaxis for hemophilia A with or without FVIII inhibitors from the HAVEN 1-4 studies
- PMID: 33512413
- PMCID: PMC8065240
- DOI: 10.1182/blood.2020009217
Long-term outcomes with emicizumab prophylaxis for hemophilia A with or without FVIII inhibitors from the HAVEN 1-4 studies
Erratum in
-
Callaghan MU, Negrier C, Paz-Priel I, et al. Long-term outcomes with emicizumab prophylaxis for hemophilia A with or without FVIII inhibitors from the HAVEN 1-4 studies. Blood. 2021;137(16):2231-2242.Blood. 2023 Oct 12;142(15):1329. doi: 10.1182/blood.2023022214. Blood. 2023. PMID: 37824158 Free PMC article. No abstract available.
Abstract
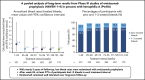
Prophylaxis with emicizumab, a subcutaneously administered bispecific humanized monoclonal antibody, promotes effective hemostasis in persons with hemophilia A (PwHAs). The primary efficacy, safety, and pharmacokinetics of emicizumab were reported previously, but long-term data were limited. Here, data from 401 pediatric and adult PwHAs with/without factor VIII (FVIII) inhibitors who were enrolled in the phase 3 HAVEN 1, HAVEN 2, HAVEN 3, and HAVEN 4 studies (NCT02622321, NCT02795767, NCT02847637, NCT03020160) have been pooled to establish a long-term efficacy, safety, and pharmacokinetics profile. Across a median efficacy period of 120.4 weeks (interquartile range, 89.0-164.4) (data cutoff 15 May 2020), the model-based treated annualized bleed rate (ABR) was 1.4 (95% confidence interval [CI], 1.1-1.7). ABRs declined and then stabilized at <1 in an analysis of 24-week treatment intervals; at weeks 121 to 144 (n = 170), the mean treated ABR was 0.7 (95% CI, 0-5.0). During weeks 121 to 144, 82.4% of participants had 0 treated bleeds, 97.6% had ≤3 treated bleeds, and 94.1% reported no treated target joint bleeds. Bleeding into target joints decreased substantially. Emicizumab was well tolerated, and no participant discontinued because of adverse events beyond the 5 previously described. This data cutoff includes the previously reported 3 thrombotic microangiopathies (one in the PwHA with fatal rectal hemorrhage) and 2 thromboembolic events, all associated with activated prothrombin complex concentrate use, as well as a myocardial infarction and a venous device occlusion. With 970.3 patient-years of exposure, emicizumab prophylaxis maintained low bleed rates in PwHAs of all ages with/without FVIII inhibitors and remains well tolerated, with no new safety concerns identified.
© 2021 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: M.U.C. has received honoraria and consulting fees from Genentech, Inc/F. Hoffmann-La Roche Ltd, Takeda, Sanofi, Pfizer, Bayer, Global Blood Therapeutics, bluebird bio, uniQure, Spark Therapeutics, Inc, BioMarin, Kedrion, Grifols, and HEMA Biologics; has received speakers’ bureau fees from Genentech, Inc/F. Hoffmann-La Roche Ltd, Takeda, Bayer, Global Blood Therapeutics, BioMarin, and Novo Nordisk; has had travel expenses paid by Genentech, Inc/F. Hoffmann-La Roche Ltd, Takeda, Sanofi, Pfizer, Bayer, Global Blood Therapeutics, uniQure, Spark Therapeutics, Inc, BioMarin, Kedrion, and HEMA Biologics; has received expert testimony fees from Genentech, Inc/F. Hoffmann-La Roche Ltd; has received research funding from Pfizer; and has stock ownership in Alnylam Pharmaceuticals. C.N. has received consultancy fees from Bayer, BioMarin, CSL Behring, Freeline, LFB Biopharmaceuticals, Novo Nordisk, Octapharma, Pfizer, F. Hoffmann-La Roche Ltd, Sanofi, Shire/Takeda, Sobi, and Spark Therapeutics, Inc; has received research funding from CSL Behring, Octapharma, Shire/Takeda, and Sobi; as has had travel expenses and accommodations paid by CSL Behring, F. Hoffmann-La Roche Ltd, and Sobi. I.P.-P. is an employee of Genentech, Inc. T.C. is an employee of, and has stock ownership in, Genentech, Inc/F. Hoffmann-La Roche Ltd. S.C. and M.L. are employees of, and have stock ownership in, F. Hoffmann-La Roche Ltd. J.M. is president of the College of Pathologists and is an employee of the University of the Witwatersrand and National Health Laboratory Service and has received honoraria, consulting fees, speakers’ bureau fees, and research funding from CSL Behring, Catalyst Biosciences, Freeline Therapeutics, Novo Nordisk, F. Hoffmann-La Roche Ltd, Sanofi, Spark Therapeutics, Inc, and Takeda. G.Y. has received honoraria from BioMarin, Genentech, Inc/F. Hoffmann-La Roche Ltd, Grifols, Kedrion, Novo Nordisk, Sanofi Genzyme, Spark Therapeutics, Inc, Takeda; has acted as a consultant for and had travel expenses paid by BioMarin, Freeline, Genentech, Inc/F. Hoffmann-La Roche Ltd, Grifols, Kedrion, Novo Nordisk, Sanofi Genzyme, Spark Therapeutics, Inc, Takeda, and UniQure; has provided expert testimony for Bayer, CSL Behring, and Genentech, Inc; and has received research funding from Grifols, Takeda, and Genentech, Inc. R.K.-J. has received honoraria and consultancy fees from Takeda, Chugai Pharmaceutical Co, Genentech, Inc/F. Hoffmann-La Roche Ltd, CSL Behring, BioMarin, and CRISPR; has been a member of the speakers’ bureau for Genentech, Inc/F. Hoffmann-La Roche Ltd. and Sanofi; and has received research funding from Pfizer, CSL Behring, and Genentech, Inc. M.E.M. has acted as a consultant and is a member of the speakers’ bureau for Bayer, BioMarin, Catalyst, CSL Behring, Grifols, Kedrion, Novo Nordisk, Octapharma, Pfizer, F. Hoffmann-La Roche Ltd, Sobi, and Takeda. M.N. and N.S.B. are employees of F. Hoffmann-La Roche Ltd. M.H. is an employee of Roche Canada; has had travel expenses paid by, and holds stock ownership in, F. Hoffmann-La Roche Ltd; and was recently employed by PRO Unlimited. M.S. has received honoraria from Chugai Pharmaceutical Co, CSL Behring, Bayer, Sanofi, Novo Nordisk, Takeda, Pfizer, Sysmex, and KM Biologics; has received research funding from Chugai Pharmaceutical Co, Bayer, Novo Nordisk, Sanofi, KM Biologics, Asahi KASEI, Sysmex, and Takeda; and holds patents with Chugai Pharmaceutical Co and Sysmex. V.J.-Y. has received reimbursement for attending symposia/congresses and/or honoraria for speaking or consulting and/or funds for research from Takeda, Bayer, CSL Behring, Grifols, BioMarin, Novo Nordisk, Sobi, F. Hoffmann-La Roche Ltd, Octapharma, and Pfizer. C.S. and E.A. are employees of, and hold stock ownership in, F. Hoffmann-La Roche Ltd. G.G.L. is a previous employee of Genentech, Inc and a current employee of Spark Therapeutics, Inc (leadership role); reports stock ownership in F. Hoffmann-La Roche Ltd; and holds patents with Baxalta, Inc. S.W.P. is an employee of the University of Michigan and is Chair of the Medical and Scientific Advisory Council to the National Hemophilia Foundation; has acted as a consultant for ApcinteX, Bayer, BioMarin, Catalyst Biosciences, CSL Behring, HEMA Biologics, Freeline, Novo Nordisk, Pfizer, Genentech, Inc/F. Hoffmann-La Roche Ltd, Sangamo Therapeutics, Sanofi, Takeda, Spark Therapeutics, Inc, and uniQure; and has received research funding from Siemens. J.O. has received honoraria, consultancy fees, and speakers’ bureau fees from Bayer, Biogen Idec, Biotest, CSL Behring, Chugai, Grifols, Novo Nordisk, Octapharma, Pfizer, F. Hoffmann-La Roche Ltd, Takeda, and Swedish Orphan Biovitrum and has received research funding from Bayer, Biotest, CSL Behring, and Takeda.
Figures



Comment in
-
In hemophilia, it just keeps getting better.Blood. 2021 Apr 22;137(16):2135-2136. doi: 10.1182/blood.2020010238. Blood. 2021. PMID: 33885708 No abstract available.
References
-
- Mannucci PM, Tuddenham EG. The hemophilias–from royal genes to gene therapy. N Engl J Med. 2001;344(23):1773-1779. - PubMed
-
- Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Treatment Guidelines Working Group on Behalf of The World Federation Of Hemophilia. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1-e47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

