Antibody-induced procoagulant platelets in severe COVID-19 infection
- PMID: 33512415
- PMCID: PMC7791311
- DOI: 10.1182/blood.2020008762
Antibody-induced procoagulant platelets in severe COVID-19 infection
Abstract
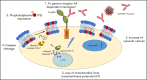
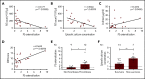
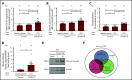
The pathophysiology of COVID-19-associated thrombosis seems to be multifactorial. We hypothesized that COVID-19 is accompanied by procoagulant platelets with subsequent alteration of the coagulation system. We investigated depolarization of mitochondrial inner transmembrane potential (ΔΨm), cytosolic calcium (Ca2+) concentration, and phosphatidylserine (PS) externalization. Platelets from COVID-19 patients in the intensive care unit (ICU; n = 21) showed higher ΔΨm depolarization, cytosolic Ca2+, and PS externalization compared with healthy controls (n = 18) and non-ICU COVID-19 patients (n = 4). Moreover, significant higher cytosolic Ca2+ and PS were observed compared with a septic ICU control group (ICU control; n = 5). In the ICU control group, cytosolic Ca2+ and PS externalization were comparable with healthy controls, with an increase in ΔΨm depolarization. Sera from COVID-19 patients in the ICU induced a significant increase in apoptosis markers (ΔΨm depolarization, cytosolic Ca2+, and PS externalization) compared with healthy volunteers and septic ICU controls. Interestingly, immunoglobulin G fractions from COVID-19 patients induced an Fcγ receptor IIA-dependent platelet apoptosis (ΔΨm depolarization, cytosolic Ca2+, and PS externalization). Enhanced PS externalization in platelets from COVID-19 patients in the ICU was associated with increased sequential organ failure assessment score (r = 0.5635) and D-dimer (r = 0.4473). Most importantly, patients with thrombosis had significantly higher PS externalization compared with those without. The strong correlations between markers for apoptosic and procoagulant platelets and D-dimer levels, as well as the incidence of thrombosis, may indicate that antibody-mediated procoagulant platelets potentially contributes to sustained increased thromboembolic risk in ICU COVID-19 patients.
© 2021 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures



Comment in
-
Another front in COVID-19's perfect storm.Blood. 2021 Feb 25;137(8):1006-1007. doi: 10.1182/blood.2020010459. Blood. 2021. PMID: 33630053 Free PMC article.
References
-
- Heemskerk JW, Mattheij NJ, Cosemans JM. Platelet-based coagulation: different populations, different functions. J Thromb Haemost. 2013;11(1):2-16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

