Antibody response in patients admitted to the hospital with suspected SARS-CoV-2 infection: results from a multicenter study across Spain
- PMID: 33512616
- PMCID: PMC7843881
- DOI: 10.1007/s10096-020-04139-5
Antibody response in patients admitted to the hospital with suspected SARS-CoV-2 infection: results from a multicenter study across Spain
Abstract
Aim: To evaluate the serological response against SARS-CoV-2 in a multicenter study representative of the Spanish COVID pandemic.
Methods: IgG and IgM + IgA responses were measured on 1466 samples from 1236 Spanish COVID-19 patients admitted to the hospital, two commercial ELISA kits (Vircell SL, Spain) based on the detection of antibodies against the viral spike protein and nucleoprotein, were used.
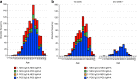
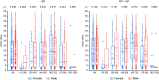
Results: Approximately half of the patients presented antibodies (56.8% were IgM + IgA positive and 43.0% were IgG positive) as soon as 2 days after the first positive PCR result. Serological test positivity increased with time from the PCR test, and 10 days after the first PCR result, 91.5% and 88.0% of the patients presented IgM + IgA and IgG antibodies, respectively.
Conclusion: The high values of sensitivity attained in the present study from a relatively early period of time after hospitalization support the use of the evaluated serological assays as supplementary diagnostic tests for the clinical management of COVID-19.
Keywords: COVID-19; Diagnosis; IgA; IgG; IgM; SARS-COV-2.
Conflict of interest statement
All authors declare no conflict of interest for the subject of the study
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

