Comparison of nine different commercially available molecular assays for detection of SARS-CoV-2 RNA
- PMID: 33512617
- PMCID: PMC7845288
- DOI: 10.1007/s10096-021-04159-9
Comparison of nine different commercially available molecular assays for detection of SARS-CoV-2 RNA
Abstract
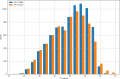
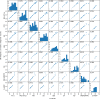
To face the COVID-19 pandemic, the need for fast and reliable diagnostic assays for the detection of SARS-CoV-2 is immense. We describe our laboratory experiences evaluating nine commercially available real-time RT-PCR assays. We found that assays differed considerably in performance and validation before routine use is mandatory.
Keywords: COVID-19; Coronavirus; Diagnostics; Infectious disease; Laboratory diagnostics; Molecular testing; Real-time PCR; SARS-CoV-2.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Konrad R, Eberle U, Dangel A, Treis B, Berger A, Bengs K, Fingerle V, Liebl B, Ackermann N, Sing A. Rapid establishment of laboratory diagnostics for the novel coronavirus SARS-CoV-2 in Bavaria, Germany, February 2020. Euro Surveill. 2020;25(9):2000173. doi: 10.2807/1560-7917.ES.2020.25.9.2000173. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

