Supporting international networks through platforms for standardised data collection-the European Registries for Rare Endocrine Conditions (EuRRECa) model
- PMID: 33512655
- PMCID: PMC7844549
- DOI: 10.1007/s12020-021-02617-0
Supporting international networks through platforms for standardised data collection-the European Registries for Rare Endocrine Conditions (EuRRECa) model
Abstract
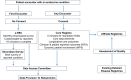
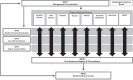
Rare endocrine pathology is manifested by either a deficiency or excess of one or more hormones. These conditions can be life-threatening and are almost universally associated with long-term morbidity. Understanding the aetiology of these conditions requires multicentre collaboration and expertise, most often across national boundaries, with the capacity for long-term follow-up. The EuRRECa (European Registries for Rare Endocrine Conditions) project ( www.eurreca.net ), funded by the EU Health Programme, aims to support the needs of the wider endocrine community by maximising the opportunity for collaboration between patients, health care professionals and researchers across Europe and beyond. At the heart of the EuRRECa collaboration is a Core Endocrine Registry that collects a core dataset for all rare endocrine conditions that are covered within Endo-ERN. The registry incorporates patient reported markers of clinical outcome and will signpost participants to high-quality, disease-specific registries. Furthermore, an electronic surveillance programme (e-REC) captures clinical activity and epidemiology for these rare conditions. EuRRECa receives guidance compliant with the highest ethical standards from Expert Working Groups that align with the Main Thematic Groups of Endo-ERN. Security, data quality and data governance are cornerstones of this platform. Clear policies that are acceptable to patients, researchers and industry for data governance coupled with widespread dissemination and knowledge exchange through closely affiliated stakeholders will ensure sustainability beyond the current lifetime of the project. This paper describes the infrastructure that has been developed, stakeholder involvement, the data fields that are captured within the registry and details on the process for using the platform.
Keywords: Databases; Endocrinology; European Reference Networks; Rare conditions; Rare diseases; Registries.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- EUCERD core recommendations on rare disease patient registration and data collection. http://www.eucerd.eu/wpcontent/uploads/2013/06/EUCERD_Recommendations_RD.... Accessed Nov 2020
-
- International Rare Disease Research Consortium. http://wwwirdirc.org. Accessed Nov 2020
-
- Gianotti S, Torreri P, Wang CM, Reihs R, Mueller H, Heslop E, Roos M, Badowska DM, dePailis F, Kodra Y, Carta C, Martin EL, Miller VR, Filocamio M, Mora M, Thompson M, Rubinstein Y, Posada de la Paz M, Monaco L, Lochmuller H, Taruscio D. The RD-Connect Registry & Biobank Finder: a tool for sharing aggregated data and metadata among rare disease researchers. Eur. J. Hum. Genet. 2018;26:631–643. doi: 10.1038/s41431-017-0085-z. - DOI - PMC - PubMed
-
- Ali SR, Bryce J, Cools M, Korbonits M, Beun JG, Taruscio D, Danne T, Dattani M, Dekkers OM, Linglart A, Netchine I, Nordenstrom A, Patocs A, Persani L, Reisch N, Smyth A, Sumnik Z, Visser WE, Hiort O, Pereira AM, Ahmed SF. The current landscape of European registries for rare endocrine conditions. Eur. J. Endocrinol. 2019;180:89–98. doi: 10.1530/EJE-18-0861. - DOI - PMC - PubMed
-
- European Registry for Rare Endocrine Conditions (EuRRECa): Core Registry Conditions Dictionary. https://eurreca.files.wordpress.com/2020/11/eurreca-core-registry-condit.... Accessed Nov 2020
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical