IVIg increases interleukin-11 levels, which in turn contribute to increased platelets, VWF and FVIII in mice and humans
- PMID: 33512707
- PMCID: PMC8062997
- DOI: 10.1111/cei.13580
IVIg increases interleukin-11 levels, which in turn contribute to increased platelets, VWF and FVIII in mice and humans
Abstract
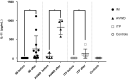
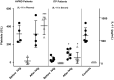
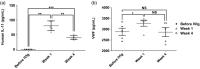
The mechanisms of action of intravenous immunoglobulins (IVIg) in autoimmune diseases are not fully understood. The fixed duration of efficacy and noncumulative effects of IVIg in immune thrombocytopenia (ITP) and acquired von Willebrand disease (AVWD) suggest other mechanisms besides immunological ones. Additionally to the peripheral destruction of platelets in ITP, their medullary hypoproduction emerged as a new paradigm with rescue of thrombopoietin receptor agonists (TPO-RA). In an ITP mouse model, interleukin (IL)-11 blood levels increase following IVIg. IL-11 stimulates the production of platelets and other haemostasis factors; recombinant IL-11 (rIL-11) is thus used as a growth factor in post-chemotherapy thrombocytopenia. We therefore hypothesized that IVIg induces IL-11 over-production, which increases platelets, VWF and factor VIII (FVIII) levels in humans and mice. First, in an ITP mouse model, we show that IVIg or rIL-11 induces a rapid increase (72 h) in platelets, FVIII and VWF levels, whereas anti-IL-11 antibody greatly decreased this effect. Secondly, we quantify for the first time in patients with ITP, AVWD, inflammatory myopathies or Guillain-Barré syndrome the dramatic IL-11 increase following IVIg, regardless of the disease. As observed in mice, platelets, VWF and FVIII levels increased following IVIg. The late evolution (4 weeks) of post-IVIg IL-11 levels overlapped with those of VWF and platelets. These data may explain thrombotic events following IVIg and open perspectives to monitor post-IVIg IL-11/thrombopoietin ratios, and to assess rIL-11 use with or without TPO-RA as megakaryopoiesis co-stimulating factors to overcome the relative hypoproduction of platelets or VWF in corresponding autoimmune diseases, besides immunosuppressant.
Keywords: Factor VIII; acquired von Willebrand disease; immune thrombocytopenia; interleukin-11; intravenous immunoglobulin.
© 2021 British Society for Immunology.
Conflict of interest statement
There are no competing interests for any author.
Figures


References
-
- Galeotti C, Kaveri SV, Bayry J. IVIG‐mediated effector functions in autoimmune and inflammatory diseases. Int Immunol 2017; 29:491–8. - PubMed
-
- Chang M, Nakagawa PA, Williams SA et al. Immune thrombocytopenic purpura (ITP) plasma and purified ITP monoclonal autoantibodies inhibit megakaryocytopoiesis in vitro . Blood 2003; 102:887–95. - PubMed
-
- Nugent D, McMillan R, Nichol JL, Slichter SJ. Pathogenesis of chronic immune thrombocytopenia: increased platelet destruction and/or decreased platelet production. Br J Haematol 2009; 146:585–96. - PubMed
-
- Rivière É, Viallard J‐F, Guy A et al. Intrinsically impaired platelet production in some patients with persistent or chronic immune thrombocytopenia. Br J Haematol 2015; 170:408–15. - PubMed
-
- Mahevas M, Van Eeckhoudt S, Moulis G et al. Autologous111 Indium‐oxinate‐labelled platelet sequestration study in patients with immune thrombocytopenia treated by thrombopoietin receptor‐agonists. Br J Haematol 2019; 186:e44–e47. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

