EVL regulates VEGF receptor-2 internalization and signaling in developmental angiogenesis
- PMID: 33512764
- PMCID: PMC7857432
- DOI: 10.15252/embr.201948961
EVL regulates VEGF receptor-2 internalization and signaling in developmental angiogenesis
Abstract
Endothelial tip cells are essential for VEGF-induced angiogenesis, but underlying mechanisms are elusive. The Ena/VASP protein family, consisting of EVL, VASP, and Mena, plays a pivotal role in axon guidance. Given that axonal growth cones and endothelial tip cells share many common features, from the morphological to the molecular level, we investigated the role of Ena/VASP proteins in angiogenesis. EVL and VASP, but not Mena, are expressed in endothelial cells of the postnatal mouse retina. Global deletion of EVL (but not VASP) compromises the radial sprouting of the vascular plexus in mice. Similarly, endothelial-specific EVL deletion compromises the radial sprouting of the vascular plexus and reduces the endothelial tip cell density and filopodia formation. Gene sets involved in blood vessel development and angiogenesis are down-regulated in EVL-deficient P5-retinal endothelial cells. Consistently, EVL deletion impairs VEGF-induced endothelial cell proliferation and sprouting, and reduces the internalization and phosphorylation of VEGF receptor 2 and its downstream signaling via the MAPK/ERK pathway. Together, we show that endothelial EVL regulates sprouting angiogenesis via VEGF receptor-2 internalization and signaling.
Keywords: Ena/VASP proteins; VEGF receptor 2 internalization and signaling; endothelial cells; sprouting angiogenesis; tip cell filopodia formation.
© 2021 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


- A, B
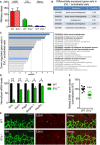
RNA sequencing of CD31 and CD34 double‐positive retinal endothelial cells from P5 wild‐type mice. (A) RNA levels (FPKM; fragments per kilobase million) of marker genes of endothelial cells (green; CD31 (gene name PECAM1), CD34, von Willebrand Factor (vWF), tyrosine‐protein kinase receptor Tie2 (TEK), VE‐cadherin (CDH5), endoglin (ENG), CD146 (MCAM) and VEGFR2 (KDR)), astrocytes (blue, GFAP), immune cells (red; T cells (CD3E), B‐cells (CD19), all leukocytes (CD45, PTPRC), and monocytes/macrophages (F4/80, EMR1)), Müller glial cells (orange; aquaporin 4 (AQP4)), neurons (yellow; retinal ganglion cells (RNA binding fox‐1 homolog 3, RBFOX3), amacrine cells (parvalbumin, PVALB), bipolar cells (PKC‐α, PRKCA), horizontal cell (calbindin, CALB1), photoreceptors (rods, CD73 (NT5E); cones, transducing (GNAT1)), and retinal pigment epithelial cells (magenta; retinal pigment epithelium‐specific 65 kDa protein (RPE65). (B) RNA levels of VASP (red), EVL (green), and Mena (blue) in the CD31 and CD34 double‐positive P5 retinal endothelial cells. Error bars represent SEM; n = 18 animals (36 retinas) from six independent litters; four independent experiments. ***P < 0.001, one‐way ANOVA with Bonferroni’s multi comparison test.
- C–E
Staining of Ena/VASP proteins in blood vessels of P5 mouse retinas. P5 wild‐type mouse retinas were fixed and stained with isolectin B4 (IB4, green) to visualize endothelial cells and VASP‐ (C), EVL‐ (D), or Mena‐specific (E) antibodies (red). Yellow color in the merged images indicates the expression of VASP and EVL proteins in endothelial cells. No Mena protein expression was detected in P5 retinal endothelial cells (E). Representative images from three independent experiments are shown. Scale bars, 10 µm.

Generation of global and tissue‐specific EVL‐deficient mice. In the knockout first alleles, a trapping element consisting of a splice acceptor (SA), the promoterless lacZ gene, a polyadenylation signal (pA), and a neomycin resistance (neo) is inserted in intron 3 of the EVL gene. Splicing (dashed line) from EVL exon 3 (gray box) to the splice acceptor of the trapping cassette induces the global disruption of the EVL gene (EVL−/−). FLP‐mediated recombination of the FRT sites (green rectangles) deletes the trapping elements and generates conditional alleles (EVLfl/fl) with loxP sites flanking the critical EVL exons 4–6. Recombination of loxP sites (red triangles) by the tamoxifen‐inducible, pdgfb‐driven Cre (Pdgfb‐iCre/ERT2) deletes EVL exons 4–6, creates a frameshift mutation, and thus generates endothelial‐specific EVL‐deficient mice (EVLΔEC).
Characterization of EVL‐specific antibodies. HEK293 cells were transfected with EVL, VASP, or Mena (CMV‐EVL, ‐VASP, ‐Mena) or MOCK (CMV‐expression plasmid without insert) and analyzed by Western blotting with EVL‐specific antibodies (left panel). Expression of VASP and Mena in the corresponding lysates was confirmed by Western blotting with anti‐VASP (middle) or anti‐Mena (right) antibodies, respectively.
Primary human dermal lymphatic endothelial cells (HDLEC), human endothelium‐derived cells (EA.hy926), and human monocytic cells (THP‐1) were lysed and analyzed by Western blotting with EVL‐specific antibodies (upper panel) or actin‐specific antibodies (lower panel).
Western blot analysis of EVL expression in lung, spleen, brain, and retina from adult wild‐type (WT), P5 wild‐type (WT P5), and adult EVL−/− (−/−) mice. EVL‐specific antibodies detected the short EVL (60 kDa) and the long EVL‐I (65 kDa) protein isoforms. MOCK and EVL‐transfected HEK cells were used as positive or negative controls, respectively. Actin was used as loading control.
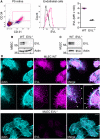
- A
Wild‐type and EVL−/− P5 retinas were digested, labeled with CD31‐, CD34‐, and EVL‐specific antibodies, and analyzed by flow cytometry. Endothelial cells were defined as CD31/CD34‐double‐positive cells (gate indicated in the left panel) and analyzed for EVL expression (middle panel). Mean fluorescence intensity (MFI) of EVL in wild‐type (magenta) and EVL−/− (black) cells is shown in the right panel. WT: n = 3, EVL−/−: n = 2; two retinas per animal. Error bars represent SEM.
- B, C
EVL protein expression in sparse/migrating wild‐type (WT) and EVL−/− mouse brain endothelial cells (MBEC) and mouse lung endothelial cells (MLEC). Actin was used as loading control. Western blots are representative of three independent experiments.
- D
MLEC from wild‐type (upper panel) and EVL−/− (lower panel) mice stimulated with 10 ng/ml VEGF were stained for actin (cyan) and EVL (magenta). Asterisks indicate focal adhesions, white arrows indicate filopodia, and white arrowheads indicate the leading edge of lamellipodia. Representative images from four independent experiments are shown. Scale bar, 20 µm.
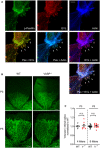
- A
EVL localizes to focal adhesions in endothelial cells. MLEC from wild‐type mice were stained for phospho‐paxilin (green) as a marker for focal adhesions, EVL (red) and actin (blue). White arrows indicate integrin‐based focal adhesions at the tips of actin stress fibers. Representative images from three independent experiments are shown. Scale bar, 10 µm.
- B, C
Postnatal retinal angiogenesis in VASP−/− mice. (B) Isolectin B4‐stained vasculature in whole mount retinas of wild‐type (WT) and global VASP−/− mice on postnatal days 3 and 5 (P3, P5) assessed by confocal microscopy. Scale bars 200 µm. (C) Analysis of the radial vascular outgrowth relative to retinal radius and normalized to wild‐type littermates. Error bars represent SEM; no significant difference was observed between the two genotypes at P3 (P > 0.999) or P5 (P > 0.999) (one‐way ANOVA with Bonferroni’s multi‐comparison test).
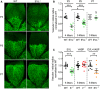
Isolectin B4 stained vasculature in whole mount retinas of wild‐type (WT) and global EVL−/− mice on postnatal days 3, 5, and 7 (P3, P5, P7) assessed by confocal microscopy. Scale bars 200 µm.
Analysis of the radial vascular outgrowth relative to retinal radius and normalized to wild‐type littermates.
Impact of individual and combined VASP/EVL deletion on sprouting angiogenesis in the postnatal mouse retina at P5 (EVL‐deficient animals (E−/−), green; VASP‐deficient animals (V−/−), red; VASP/EVL‐double deficient animals (EV−/−), orange).
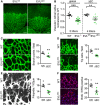
- A, B
Radial outgrowth of the retinal vasculature; in (B) global EVL−/− mice are shown for comparison; 4–5 independent experiments.
- C
Quantification of the branch points in a field of 170 μm × 300 μm directly behind the vascular front.
- D
Tipp cell numbers per field of view (387.5 × 387.5 µm).
- E
Filopodia numbers normalized to the length of the angiogenic front.
- F
Endothelial cell proliferation analyzed by BrdU incorporation (red) of ERG‐positive (blue) endothelial nuclei.
RNA levels (FPKM) of VASP, EVL, and Mena in endothelial cells from wild‐type or EVL−/− retinas.
Pathway analysis (metascape.org) was used to identify differentially expressed gene sets in EVL−/− retinal endothelial cells. Selected gene sets involved in vessel branching are shown.
Pathway analysis of all under‐represented gene sets in EVL−/− endothelial cells.
Analysis of mRNA levels of Esm1, Ptgs2 (cyclooxygenase 2), serpine 1 and paxillin in EVL‐deficient MLEC relative to WT controls set to 100. n = 3–4 independent experiments, error bars represent SEM, one‐sample t‐test, *P < 0.05; **P < 0.01.
ESM1 protein expression in P5 retinas of wild‐type (WT) and global EVL−/− mice. Retinas were fixed and stained with isolectin B4 (IB4, green) to visualize endothelial cells and antibodies directed against the tip cell marker ESM1 (red). Representative images from three independent experiments are shown. Scale bars, 100 µm.
Analysis of retinal ESM1 protein levels normalized to wild‐type littermates. Error bars represent SEM; ***P < 0.001, unpaired Student’s t‐test; three different litters.
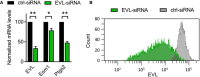
Analysis of mRNA levels of EVL, Esm1, and Ptgs2 in HUVEC transfected with EVL‐specific or control siRNA. n = 3 independent experiments, error bars represent SEM, one‐sample t‐test, *P < 0.05; **P < 0.01.
FACS analysis of EVL protein expression in HUVEC transfected with EVL‐specific or control siRNA. One representative image of three independent experiments is shown.
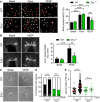
Proliferation of WT and EVL−/− MLEC was assessed by BrdU incorporation. The number of BrdU‐positive cells (red) was normalized to the total number of cells (white). Eight fields of view per condition and genotype for each of 5 independent WT and EVL−/− cell batches; VEGF stimulation (10 ng/ml) error bars represent SEM; **P < 0.01, ***P < 0.001, # P = 0.171 non‐significant vs. Basal EVL−/−, one‐way ANOVA with Bonferroni’s multi comparison test; scale bar = 50 µm.
Cumulative length of CD31‐positive sprouts from WT and EVL−/− aortic rings embedded in collagen after 5 days without (basal) or with VEGF stimulation (30 ng/ml). 7 WT and 6 EVL−/− adult animals from three different litters, each; 3 aortic rings per animal and condition; error bars represent SEM; **P < 0.01; ***P < 0.01; # P = 0.543 non‐significant vs. basal EVL−/−, one‐way ANOVA with Bonferroni’s multi comparison test; scale bar, 250 µm.
Sprouting of WT and EVL−/− primary MLEC spheres in the absence (Basal) or presence of VEGF (50 ng/ml); asterisks indicate sprouts; scale bars, 100 µm.
Solid bars represent proportion of WT and EVL−/− MLEC spheres forming sprouts in the absence (−) or presence (+) of VEGF. n‐numbers are indicated; n.s. non‐significant (P = 0.557); ***P < 0.001, Fisher’s exact test.
Quantification of MLEC sphere sprout length without or with VEGF. Dots represent individual sprouts. Horizontal lines represent mean, error bars represent SEM. n = 20–30 beads (from three independent experiments). ***P < 0.001, n.s. non‐significant (P = 0.543), one‐way ANOVA with Bonferroni’s multi comparison test.
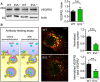
VEGFR2 protein levels in wild‐type (WT) and EVL‐deficient (EVL−/−) endothelial cells. Actin was used as loading control. The lower panel shows quantification of VEGFR2 to actin ratios from five independent cell batches; error bars represent SEM; n.s. non‐significant (P = 0.388), unpaired Student’s t‐test.
Antibody feeding assay to monitor VEGFR2 internalization after VEGF stimulation. Left panel: schematic diagram showing the two step staining procedure of surface and internalized VEGFR2 antibodies, which are displayed in yellow and green in the merged confocal images of WT and EVL−/− endothelial cells (middle panel), respectively; scale bar, 10 µm. The bar graphs show quantification of the number of VEGFR2 clusters and the ratio of internalized to total VEGFR2 normalized to wild‐type; n = 4–6 independent cell batches, error bars represent SEM; **P < 0.01, ***P < 0.001, unpaired Student’s t‐test.
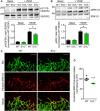
Western blots and quantification of VEGFR2 phosphorylation levels relative to total VEGFR2 levels in WT and EVL−/− endothelial cells under basal and VEGF‐stimulated conditions (80 ng/ml); n = 5 independent cell batches, error bars represent SEM; *P < 0.05, one‐way ANOVA with Bonferroni’s multi comparison test.
Western blots and quantification of ERK1/2 phosphorylation levels relative to total ERK levels in WT and EVL−/− endothelial cells under basal and VEGF‐stimulated conditions (80 ng/ml); n = 6 independent cell batches, error bars represent SEM; *P < 0.05, one‐way ANOVA with Bonferroni’s multi comparison test.
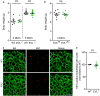
ERK1/2 phosphorylation in P5 retinas of wild‐type (WT) and global EVL−/− mice. Retinas were fixed and stained with isolectin B4 (IB4, green) to visualize endothelial cells and antibodies directed against phospho‐ERK1/2 (red). Representative images from three independent experiments are shown. Scale bars, 100 µm.
Analysis of endothelial ERK1/2 phosphorylation normalized to wild‐type littermates. Error bars represent SEM; ***P < 0.001, unpaired Student’s t‐test; two different litters.
- A, B
Body weight of wild‐type and EVL‐deficient mice. (A) Body weight of wild‐type and global EVL‐deficient mice at postnatal days 3 and 5. No significant difference in body weight was observed between the two genotypes at P3 (P > 0.999) or P5 (P > 0.999). One‐way ANOVA with Bonferroni’s multi comparison test; 4 and 5 different litters, respectively, error bars represent SEM. (B) Endothelial cell‐specific EVL knockout mice (EVLΔEC) and littermate controls (EVLfl/fl) at P5. No significant difference in body weight was observed between the two groups (P = 0.753). Unpaired Student’s t‐test. Error bars represent SEM.
- C
Ki67 expression in P7 retinas of wild‐type (WT) and global EVL−/− mice. Retinas were fixed and stained with isolectin B4 (IB4, green) to visualize endothelial cells and antibodies directed against Ki67 (red) to visualize proliferation. Representative images from three independent experiments are shown. Scale bars, 100 µm.
- D
Analysis of proliferation of wild‐type and EVL−/− retinal endothelial cells at P7 by Ki67 immunofluorescence microscopy. Data are normalized to the mean WT value. No significant difference in Ki67‐positive endothelial cells was observed between the two groups (P = 0.386). Unpaired Student’s t‐test; two litters. Error bars represent SEM.
References
-
- Ahern‐Djamali SM, Comer AR, Bachmann C, Kastenmeier AS, Reddy SK, Beckerle MC, Walter U, Hoffmann FM (1998) Mutations in Drosophila enabled and rescue by human vasodilator‐stimulated phosphoprotein (VASP) indicate important functional roles for Ena/VASP homology domain 1 (EVH1) and EVH2 domains. Mol Biol Cell 9: 2157–2171 - PMC - PubMed
-
- Baker M, Robinson SD, Lechertier T, Barber PR, Tavora B, D'Amico G, Jones DT, Vojnovic B, Hodivala‐Dilke K (2012) Use of the mouse aortic ring assay to study angiogenesis. Nat Protoc 7: 89–104 - PubMed
-
- Banu N, Teichman J, Dunlap‐Brown M, Villegas G, Tufro A (2006) Semaphorin 3C regulates endothelial cell function by increasing integrin activity. FASEB J 20: 2150–2152 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

