Phase I Study of Elacestrant (RAD1901), a Novel Selective Estrogen Receptor Degrader, in ER-Positive, HER2-Negative Advanced Breast Cancer
- PMID: 33513026
- PMCID: PMC8078341
- DOI: 10.1200/JCO.20.02272
Phase I Study of Elacestrant (RAD1901), a Novel Selective Estrogen Receptor Degrader, in ER-Positive, HER2-Negative Advanced Breast Cancer
Abstract
Purpose: This phase I study (RAD1901-005; NCT02338349) evaluated elacestrant, an investigational oral selective estrogen receptor degrader (SERD), in heavily pretreated women with estrogen receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer, including those with estrogen receptor gene alpha (ESR1) mutation. The primary objective was to determine the maximum tolerated dose and/or recommended phase II dose (RP2D).
Methods: The study consisted of a 3 + 3 design (elacestrant capsules) followed by expansion at RP2D (400-mg capsules, then 400-mg tablets) for the evaluation of safety and antitumor activity. Elacestrant was taken once daily until progression or intolerability.
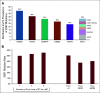
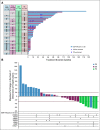
Results: Of 57 postmenopausal women enrolled, 50 received RP2D (400 mg once daily): median age, 63 years; median three prior anticancer therapies, including cyclin-dependent kinase 4,6 inhibitors (CDK4/6i; 52%), SERD (52%), and ESR1 mutation (circulating tumor DNA; 50%). No dose-limiting toxicities occurred; the most common adverse events at RP2D (400-mg tablet; n = 24) were nausea (33.3%) and increased blood triglycerides and decreased blood phosphorus (25.0% each). Most adverse events were grade 1-2 in severity. The objective response rate was 19.4% (n = 31 evaluable patients receiving RP2D), 15.0% in patients with prior SERD, 16.7% in patients with prior CDK4/6i, and 33.3% in patients with ESR1 mutation (n = 5/15). The clinical benefit rate (24-week) was 42.6% overall (n = 47 patients receiving RP2D), 56.5% (n = 23, ESR1 mutation), and 30.4% (n = 23, prior CDK4/6i). Elacestrant clinical benefit was associated with decline in ESR1 mutant allele fraction.
Conclusion: Elacestrant 400 mg orally once daily has an acceptable safety profile and demonstrated single-agent activity with confirmed partial responses in heavily pretreated patients with estrogen receptor-positive metastatic breast cancer. Notably, responses were observed in patients with ESR1 mutation as well as those with prior CDK4/6i and prior SERD. A phase III trial investigating elacestrant versus standard endocrine therapy is ongoing.
Conflict of interest statement
Figures


References
-
- Fribbens C O'Leary B Kilburn L, et al. : Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol 34:2961-2968, 2016 - PubMed
-
- Bihani T Patel HK Arlt H, et al. : Elacestrant (RAD1901), a selective estrogen receptor degrader (SERD), has antitumor activity in multiple ER+ breast cancer patient-derived xenograft models. Clin Cancer Res 23:4793-4804, 2017 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

