Evaluation of Sustained Minimal Residual Disease Negativity With Daratumumab-Combination Regimens in Relapsed and/or Refractory Multiple Myeloma: Analysis of POLLUX and CASTOR
- PMID: 33513030
- PMCID: PMC8078259
- DOI: 10.1200/JCO.20.01814
Evaluation of Sustained Minimal Residual Disease Negativity With Daratumumab-Combination Regimens in Relapsed and/or Refractory Multiple Myeloma: Analysis of POLLUX and CASTOR
Abstract
Purpose: In relapsed and/or refractory multiple myeloma, daratumumab reduced the risk of progression or death by > 60% in POLLUX (daratumumab/lenalidomide/dexamethasone [D-Rd]) and CASTOR (daratumumab/bortezomib/dexamethasone [D-Vd]). Minimal residual disease (MRD) is a sensitive measure of disease control. Sustained MRD negativity and outcomes were evaluated in these studies.
Methods: MRD was assessed via next-generation sequencing (10-5) at suspected complete response (CR), 3 and 6 months following confirmed CR (POLLUX), 6 and 12 months following the first dose (CASTOR), and every 12 months post-CR in both studies. Sustained MRD negativity (≥ 6 or ≥ 12 months) was evaluated in the intention-to-treat (ITT) and ≥ CR populations.
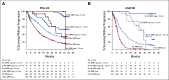
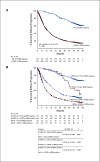
Results: The median follow-up was 54.8 months in POLLUX and 50.2 months in CASTOR. In the ITT population, MRD-negativity rates were 32.5% versus 6.7% for D-Rd versus lenalidomide and dexamethasone (Rd) and 15.1% versus 1.6% for D-Vd versus bortezomib and dexamethasone (Vd; both P < .0001). Higher MRD negativity rates were achieved in ≥ CR patients in POLLUX (D-Rd, 57.4%; Rd, 29.2%; P = .0001) and CASTOR (D-Vd, 52.8%; Vd, 17.4%; P = .0035). More patients in the ITT population achieved sustained MRD negativity ≥ 6 months with D-Rd versus Rd (20.3% v 2.1%; P < .0001) and D-Vd versus Vd (10.4% v 1.2%; P < .0001), and ≥ 12 months with D-Rd versus Rd (16.1% v 1.4%; P < .0001) and D-Vd versus Vd (6.8% v 0%). Similar results for sustained MRD negativity were observed among ≥ CR patients. More patients in the daratumumab-containing arms achieved MRD negativity and sustained MRD negativity, which were associated with prolonged progression-free survival.
Conclusion: Daratumumab-based combinations induce higher rates of sustained MRD negativity versus standard of care, which are associated with durable remissions and prolonged clinical outcomes.
Trial registration: ClinicalTrials.gov NCT02136134 NCT02076009.
Figures


References
-
- Kumar S Paiva B Anderson KC, et al. : International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 17:e328-e346, 2016 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

