Causal network inference from gene transcriptional time-series response to glucocorticoids
- PMID: 33513136
- PMCID: PMC7875426
- DOI: 10.1371/journal.pcbi.1008223
Causal network inference from gene transcriptional time-series response to glucocorticoids
Abstract
Gene regulatory network inference is essential to uncover complex relationships among gene pathways and inform downstream experiments, ultimately enabling regulatory network re-engineering. Network inference from transcriptional time-series data requires accurate, interpretable, and efficient determination of causal relationships among thousands of genes. Here, we develop Bootstrap Elastic net regression from Time Series (BETS), a statistical framework based on Granger causality for the recovery of a directed gene network from transcriptional time-series data. BETS uses elastic net regression and stability selection from bootstrapped samples to infer causal relationships among genes. BETS is highly parallelized, enabling efficient analysis of large transcriptional data sets. We show competitive accuracy on a community benchmark, the DREAM4 100-gene network inference challenge, where BETS is one of the fastest among methods of similar performance and additionally infers whether causal effects are activating or inhibitory. We apply BETS to transcriptional time-series data of differentially-expressed genes from A549 cells exposed to glucocorticoids over a period of 12 hours. We identify a network of 2768 genes and 31,945 directed edges (FDR ≤ 0.2). We validate inferred causal network edges using two external data sources: Overexpression experiments on the same glucocorticoid system, and genetic variants associated with inferred edges in primary lung tissue in the Genotype-Tissue Expression (GTEx) v6 project. BETS is available as an open source software package at https://github.com/lujonathanh/BETS.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: BEE is on the SAB for Freenome, Celsius Therapeutics, and Creyon Bio; is a consultant for Freenome; and was an employee of Genomics plc during a year of absence from Princeton University.
Figures

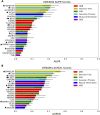



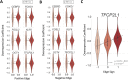

References
-
- Bernardo J, Bayarri M, Berger J, Dawid A, Heckerman D, Smith A, et al. Bayesian factor regression models in the “large p, small n” paradigm. Bayesian Statistics. 2003;7:733–742.
-
- Bühlmann P, Kalisch M, Meier L. High-dimensional statistics with a view toward applications in biology. Annual Review of Statistics and Its Application. 2014;1:255–278. 10.1146/annurev-statistics-022513-115545 - DOI
-
- Robinson JW, Hartemink AJ. Learning non-stationary dynamic Bayesian networks. Journal of Machine Learning Research. 2010;11(Dec):3647–3680.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

