The growth of siRNA-based therapeutics: Updated clinical studies
- PMID: 33513339
- PMCID: PMC8187268
- DOI: 10.1016/j.bcp.2021.114432
The growth of siRNA-based therapeutics: Updated clinical studies
Abstract
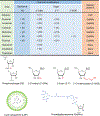
More than two decades after the natural gene-silencing mechanism of RNA interference was elucidated, small interfering RNA (siRNA)-based therapeutics have finally broken into the pharmaceutical market. With three agents already approved and many others in advanced stages of the drug development pipeline, siRNA drugs are on their way to becoming a standard modality of pharmacotherapy. The majority of late-stage candidates are indicated for rare or orphan diseases, whose patients have an urgent need for novel and effective therapies. Additionally, there are agents that have the potential to meet the need of a broader population. Inclisiran, for instance, is being developed for hypercholesterolemia and has shown benefit in patients who are uncontrolled even after maximal statin therapy. This review provides a brief overview of mechanisms of siRNA action, physiological barriers to its delivery and activity, and the most common chemical modifications and delivery platforms used to overcome these barriers. Furthermore, this review presents comprehensive profiles of the three approved siRNA drugs (patisiran, givosiran, and lumasiran) and the seven other siRNA candidates in Phase 3 clinical trials (vutrisiran, nedosiran, inclisiran, fitusiran, teprasiran, cosdosiran, and tivanisiran), summarizing their modifications and delivery strategies, disease-specific mechanisms of action, updated clinical trial status, and future outlooks.
Keywords: Clinical trial; Drug development; FDA approval; siRNAs.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors declare that there are no conflicts of interest.
Figures
References
-
- Fire A, et al., Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature, 1998. 391(6669): p. 806–11. - PubMed
-
- Setten RL, Rossi JJ, and Han SP, The current state and future directions of RNAi-based therapeutics. Nat Rev Drug Discov, 2019. 18(6): p. 421–446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical