Optimisation of Epoxide Ring-Opening Reaction for the Synthesis of Bio-Polyol from Palm Oil Derivative Using Response Surface Methodology
- PMID: 33513686
- PMCID: PMC7865885
- DOI: 10.3390/molecules26030648
Optimisation of Epoxide Ring-Opening Reaction for the Synthesis of Bio-Polyol from Palm Oil Derivative Using Response Surface Methodology
Abstract
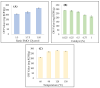
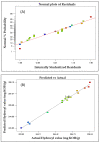
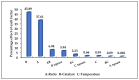
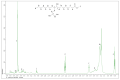
The development of bio-polyol from vegetable oil and its derivatives is gaining much interest from polyurethane industries and academia. In view of this, the availability of methyl oleate derived from palm oil, which is aimed at biodiesel production, provides an excellent feedstock to produce bio-polyol for polyurethane applications. In this recent study, response surface methodology (RSM) with a combination of central composite rotatable design (CCRD) was used to optimise the reaction parameters in order to obtain a maximised hydroxyl value (OHV). Three reaction parameters were selected, namely the mole ratio of epoxidised methyl oleate (EMO) to glycerol (1:5-1:10), the amount of catalyst loading (0.15-0.55%) and reaction temperature (90-150 °C) on a response variable as the hydroxyl value (OHV). The analysis of variance (ANOVA) indicated that the quadratic model was significant at 98% confidence level with (p-value > 0.0001) with an insignificant lack of fit and the regression coefficient (R2) was 0.9897. The optimum reaction conditions established by the predicted model were: 1:10 mole ratio of EMO to glycerol, 0.18% of catalyst and 120 °C reaction temperature, giving a hydroxyl value (OHV) of 306.190 mg KOH/g for the experimental value and 301.248 mg KOH/g for the predicted value. This result proves that the RSM model is capable of forecasting the relevant response. FTIR analysis was employed to monitor the changes of functional group for each synthesis and the confirmation of this finding was analysed by NMR analysis. The viscosity and average molecular weight (MW) were 513.48 mPa and 491 Da, respectively.
Keywords: central composite rotatable design (CCRD); epoxidation reaction; hydroxyl value; mechanism; methyl oleate; nuclear magnetic resonance (NMR).
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Cui S., Liu Z., Li Y. Bio-polyols synthesized from crude glycerol and applications on polyurethane wood adhesives. Ind. Crops Prod. 2017;108:798–805. doi: 10.1016/j.indcrop.2017.07.043. - DOI
-
- Saalah S., Abdullah L.C., Aung M.M., Salleh M.Z., Awang Biak D.R., Basri M., Jusoh E.R. Waterborne polyurethane dispersions synthesized from jatropha oil. Ind. Crops Prod. 2015;64:194–200. doi: 10.1016/j.indcrop.2014.10.046. - DOI
-
- Prociak A., Malewska E., Kurańska M., Bąk S., Budny P. Flexible polyurethane foams synthesized with palm oil-based bio-polyols obtained with the use of different oxirane ring opener. Ind. Crops Prod. 2018;115:69–77. doi: 10.1016/j.indcrop.2018.02.008. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

