Glucagon-Like Peptide-1 Receptor Agonists for Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: An Updated Meta-Analysis of Randomized Controlled Trials
- PMID: 33513761
- PMCID: PMC7911747
- DOI: 10.3390/metabo11020073
Glucagon-Like Peptide-1 Receptor Agonists for Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: An Updated Meta-Analysis of Randomized Controlled Trials
Abstract
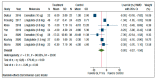
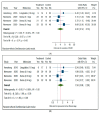
To assess the efficacy of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) for treatment of nonalcoholic fatty liver disease (NAFLD) or steatohepatitis (NASH), we performed a systematic review and meta-analysis of randomized controlled trials (RCTs). Three large electronic databases were systematically searched (up to 15 December 2020) to identify placebo-controlled or active-controlled RCTs using different GLP-1 RAs. We included eleven placebo-controlled or active-controlled phase-2 RCTs (involving a total of 936 middle-aged individuals) that used liraglutide (n = 6 RCTs), exenatide (n = 3 RCTs), dulaglutide (n = 1 RCT) or semaglutide (n = 1 RCT) to specifically treat NAFLD or NASH, detected by liver biopsy (n = 2 RCTs) or imaging techniques (n = 9 RCTs). Compared to placebo or reference therapy, treatment with GLP-1 RAs for a median of 26 weeks was associated with significant reductions in the absolute percentage of liver fat content on magnetic resonance-based techniques (pooled weighted mean difference: -3.92%, 95% confidence intervals (CI) -6.27% to -1.56%) and serum liver enzyme levels, as well as with greater histological resolution of NASH without worsening of liver fibrosis (pooled random-effects odds ratio 4.06, 95% CI 2.52-6.55; for liraglutide and semaglutide only). In conclusion, treatment with GLP-1 RAs (mostly liraglutide and semaglutide) is a promising treatment option for NAFLD or NASH that warrants further investigation.
Keywords: GLP-1 receptor agonists; NAFLD; NASH; dulaglutide; exenatide; liraglutide; nonalcoholic fatty liver disease; nonalcoholic steatohepatitis; semaglutide; type 2 diabetes mellitus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. - DOI - PubMed
-
- Lonardo A., Nascimbeni F., Targher G., Bernardi M., Bonino F., Bugianesi E., Casini A., Gastaldelli A., Marchesini G., Marra F., et al. AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions. Dig. Liver Dis. 2017;49:471–483. doi: 10.1016/j.dld.2017.01.147. - DOI - PubMed
-
- Lonardo A., Bellentani S., Argo C.K., Ballestri S., Byrne C.D., Caldwell S.H., Cortez-Pinto H., Grieco A., Machado M.V., Miele L., et al. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Dig. Liver Dis. 2015;47:997–1006. doi: 10.1016/j.dld.2015.08.004. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

