Safety and Efficacy of Probiotic Supplementation in Reducing the Incidence of Infections and Modulating Inflammation in the Elderly with Feeding Tubes: A Pilot, Double-Blind, Placebo-Controlled Study, "IntegPRO"
- PMID: 33513820
- PMCID: PMC7911800
- DOI: 10.3390/nu13020391
Safety and Efficacy of Probiotic Supplementation in Reducing the Incidence of Infections and Modulating Inflammation in the Elderly with Feeding Tubes: A Pilot, Double-Blind, Placebo-Controlled Study, "IntegPRO"
Abstract
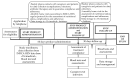
A double-blind, placebo-controlled study was performed in a sample of geriatric patients treated with home enteral nutrition (HEN) to analyze the efficacy of a probiotic supplement Proxian®, which contains Lactiplantibacillus plantarum LP01 (LMG P-21021), Lentilactobacillus buchneri Lb26 (DSM 16341), Bifidobacterium animalis subsp. lactis BS01 (LMG P-21384), and is enriched with zinc (Zn) and selenium (Se), in reducing the incidence of infections and modulating inflammation. Thirty-two subjects were enrolled (mean age 79.7 ± 10.3 years), 16 in the intervention group, 16 controls. They received Proxian® or placebo for 60 days. Patients were assessed at baseline (t0) and 60 (t1) and 90 (t2) days after the beginning. Infections were detected by information regarding their clinical manifestations and the incidence of antibiotic therapy. Levels of C-reactive protein (CRP) were measured to study inflammation. Information on bowel function, nutritional status and testimonials regarding the feasibility of administration of the product were collected. Differences between the two groups in number of infections (25% intervention group vs. 44% controls), antibiotic therapies (12% vs. 37%) and modulation of CRP levels (median CRP moved from 0.95 mg/L (t0), to 0.6 (t1) and 0.7 (t2) in intervention group vs. 0.7 mg/L, 0.5 and 0.7 in controls) did not reach statistical significance. No significant changes in bowel function and nutritional status were found. Caregivers' adherence was 100%. Results of this "IntegPRO" study showed that Proxian® is potentially safe, easy to administer and promising for further studies but it appears not to change the incidence of infections or modulate inflammation in elderly treated with HEN. The utility of Proxian® in reducing the incidence of infections and modulating inflammation in these subjects needs to be investigated by a larger multi-center clinical trial, and by using additional analyses on inflammatory markers and markers of infections.
Keywords: enteral nutrition; geriatric patients; inflammation; probiotics.
Conflict of interest statement
The authors declare no conflict of interest. The authors Angela Amoruso and Marco Pane, are employees of the company Probiotical S.p.a., Novara, Italy, which provided the active product and covered the expenses for blood tests, and did not participate in the study design, the drafting of results and conclusions.
Figures


References
-
- Minihane A.M., Vinoy S., Russell W.R., Baka A., Roche H.M., Tuohy K.M., Teeling J.L., Blaak E.E., Fenech M., Vauzour D., et al. Low grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015;114:999–1012. doi: 10.1017/S0007114515002093. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

