Cytokine-Coding Oncolytic Adenovirus TILT-123 Is Safe, Selective, and Effective as a Single Agent and in Combination with Immune Checkpoint Inhibitor Anti-PD-1
- PMID: 33513935
- PMCID: PMC7911972
- DOI: 10.3390/cells10020246
Cytokine-Coding Oncolytic Adenovirus TILT-123 Is Safe, Selective, and Effective as a Single Agent and in Combination with Immune Checkpoint Inhibitor Anti-PD-1
Abstract
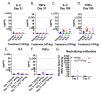
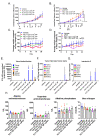
Oncolytic viruses provide a biologically multi-faceted treatment option for patients who cannot be cured with currently available treatment options. We constructed an oncolytic adenovirus, TILT-123, to support T-cell therapies and immune checkpoint inhibitors in solid tumors. Adenoviruses are immunogenic by nature, are easy to produce in large quantities, and can carry relatively large transgenes. They are the most commonly used gene therapy vectors and are well tolerated in patients. TILT-123 expresses two potent cytokines, tumor necrosis factor alpha and interleukin-2, to stimulate especially the T-cell compartment in the tumor microenvironment. Before entering clinical studies, the safety and biodistribution of TILT-123 was studied in Syrian hamsters and in mice. The results show that TILT-123 is safe in animals as monotherapy and in combination with an immune checkpoint inhibitor anti-PD-1. The virus treatment induces acute changes in circulating immune cell compartments, but the levels return to normal by the middle of the treatment period. The virus is rapidly cleared from healthy tissues, and it does not cause damage to vital organs. The results support the initiation of a phase 1 dose-escalation trial, where melanoma patients receiving a tumor-infiltrating lymphocyte therapy are treated with TILT-123 (NCT04217473).
Keywords: adenovirus; biodistribution; immunotherapy; oncolytic virus; safety.
Conflict of interest statement
R.H., R.K., and S.S. are employees of TILT Biotherapeutics Ltd. A.H., J.S., and V.C-C are employees and shareholders of TILT Biotherapeutics Ltd. A.H is a shareholder of Targovax ASA.
Figures





References
-
- Ranki T., Pesonen S., Hemminki A., Partanen K., Kairemo K., Alanko T., Lundin J., Linder N., Turkki R., Ristimaki A., et al. Phase I study with ONCOS-102 for the treatment of solid tumors-an evaluation of clinical response and exploratory analyses of immune markers. J. Immunother Cancer. 2016;4:1–18. doi: 10.1186/s40425-016-0121-5. - DOI - PMC - PubMed
-
- Lang F.F., Conrad C., Gomez-Manzano C., Yung W.K.A., Sawaya R., Weinberg J.S., Prabhu S.S., Rao G., Fuller G.N., Aldape K.D., et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018 doi: 10.1200/JCO.2017.75.8219. - DOI - PMC - PubMed
-
- Nemunaitis J., Tong A.W., Nemunaitis M., Senzer N., Phadke A.P., Bedell C., Adams N., Zhang Y.A., Maples P.B., Chen S., et al. A phase I study of telomerase-specific replication competent oncolytic adenovirus (telomelysin) for various solid tumors. Mol. Ther. 2010;18:429–434. doi: 10.1038/mt.2009.262. - DOI - PMC - PubMed
-
- Eriksson E., Milenova I., Wenthe J., Stahle M., Leja-Jarblad J., Ullenhag G., Dimberg A., Moreno R., Alemany R., Loskog A. Shaping the Tumor Stroma and Sparking Immune Activation by CD40 and 4-1BB Signaling Induced by an Armed Oncolytic Virus. Clin. Cancer Res. 2017;23:5846–5857. doi: 10.1158/1078-0432.CCR-17-0285. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

