Recent Advances in the Synthesis of β-Carboline Alkaloids
- PMID: 33513936
- PMCID: PMC7866041
- DOI: 10.3390/molecules26030663
Recent Advances in the Synthesis of β-Carboline Alkaloids
Abstract
β-Carboline alkaloids are a remarkable family of natural and synthetic indole-containing heterocyclic compounds and they are widely distributed in nature. Recently, these alkaloids have been in the focus of interest, thanks to their diverse biological activities. Their pharmacological activity makes them desirable as sedative, anxiolytic, hypnotic, anticonvulsant, antitumor, antiviral, antiparasitic or antimicrobial drug candidates. The growing potential inherent in them encourages many researchers to address the challenges of the synthesis of natural products containing complex β-carboline frameworks. In this review, we describe the recent developments in the synthesis of β-carboline alkaloids and closely related derivatives through selected examples from the last 5 years. The focus is on the key steps with improved procedures and synthetic approaches. Furthermore the pharmacological potential of the alkaloids is also highlighted.
Keywords: bioactive molecules; natural products; total synthesis; β-carboline.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









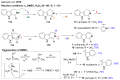





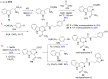
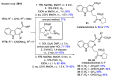

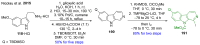
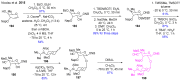










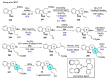







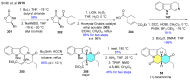
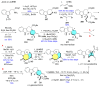









References
-
- Smirnova O.B., Golovko T.V., Granik V.G. Carbolines. Part 2: Comparison of some of the properties of α-, γ-, and δ-carbolines. Pharm. Chem. J. 2011;45:389–400. doi: 10.1007/s11094-011-0641-8. - DOI
-
- Singh V., Batra S. 1-Formyl-9H-β-carboline: A useful scaffold for synthesizing substituted and fused β-carbolines. Curr. Org. Synth. 2012;9:513–528. doi: 10.2174/157017912802651465. - DOI
-
- Casal S. Coffee: Consumption and Health Implications. The Royal Society of Chemistry; Croydon, UK: 2019. Chapter 20. Potential effects of β-carbolines on human health; pp. 461–468.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

