Targeting Lysyl Oxidase Family Meditated Matrix Cross-Linking as an Anti-Stromal Therapy in Solid Tumours
- PMID: 33513979
- PMCID: PMC7865543
- DOI: 10.3390/cancers13030491
Targeting Lysyl Oxidase Family Meditated Matrix Cross-Linking as an Anti-Stromal Therapy in Solid Tumours
Abstract
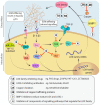
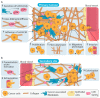
The lysyl oxidase (LOX) family of enzymes are a major driver in the biogenesis of desmoplastic matrix at the primary tumour and secondary metastatic sites. With the increasing interest in and development of anti-stromal therapies aimed at improving clinical outcomes of cancer patients, the Lox family has emerged as a potentially powerful clinical target. This review examines how lysyl oxidase family dysregulation in solid cancers contributes to disease progression and poor patient outcomes, as well as an evaluation of the preclinical landscape of LOX family targeting therapeutics. We also discuss the suitability of the LOX family as a diagnostic and/or prognostic marker in solid tumours.
Keywords: anti-stromal targeting; cancer; extracellular matrix; lysyl oxidases; tumorigenesis; tumour microenvironment.
Conflict of interest statement
T.R.C. and J.L.C. are engaged in a collaborative research project with Pharmaxis Ltd., a company with ownership of PXS-5120A, PXS-5153A, PXS-S1A/C and PXS-5382A discussed in this review.
Figures


Similar articles
-
Targeting the lysyl oxidases in tumour desmoplasia.Biochem Soc Trans. 2019 Dec 20;47(6):1661-1678. doi: 10.1042/BST20190098. Biochem Soc Trans. 2019. PMID: 31754702 Review.
-
Lysyl Oxidase (LOX) Family Proteins: Key Players in Breast Cancer Occurrence and Progression.J Cancer. 2024 Aug 13;15(16):5230-5243. doi: 10.7150/jca.98688. eCollection 2024. J Cancer. 2024. PMID: 39247609 Free PMC article. Review.
-
Lysyl Oxidase and the Tumor Microenvironment.Int J Mol Sci. 2016 Dec 29;18(1):62. doi: 10.3390/ijms18010062. Int J Mol Sci. 2016. PMID: 28036074 Free PMC article. Review.
-
Systematic Characterisation and Analysis of Lysyl Oxidase Family Members as Drivers of Tumour Progression and Multiple Drug Resistance.J Cell Mol Med. 2025 Apr;29(7):e70536. doi: 10.1111/jcmm.70536. J Cell Mol Med. 2025. PMID: 40179101 Free PMC article.
-
Lysyl oxidase, extracellular matrix remodeling and cancer metastasis.Cancer Microenviron. 2012 Dec;5(3):261-73. doi: 10.1007/s12307-012-0105-z. Epub 2012 Apr 13. Cancer Microenviron. 2012. PMID: 22528876 Free PMC article.
Cited by
-
Novel approaches to target the microenvironment of bone metastasis.Nat Rev Clin Oncol. 2021 Aug;18(8):488-505. doi: 10.1038/s41571-021-00499-9. Epub 2021 Apr 19. Nat Rev Clin Oncol. 2021. PMID: 33875860 Review.
-
Tumor-associated fibrosis: a unique mechanism promoting ovarian cancer metastasis and peritoneal dissemination.Cancer Metastasis Rev. 2024 Sep;43(3):1037-1053. doi: 10.1007/s10555-024-10169-8. Epub 2024 Mar 28. Cancer Metastasis Rev. 2024. PMID: 38546906 Free PMC article. Review.
-
Protein disulfide isomerase A3 activity promotes extracellular accumulation of proteins relevant to basal breast cancer outcomes in human MDA-MB-A231 breast cancer cells.Am J Physiol Cell Physiol. 2023 Jan 1;324(1):C113-C132. doi: 10.1152/ajpcell.00445.2022. Epub 2022 Nov 14. Am J Physiol Cell Physiol. 2023. PMID: 36374169 Free PMC article.
-
c-Src-induced vascular malformations require localised matrix degradation at focal adhesions.J Cell Sci. 2024 Jul 1;137(13):jcs262101. doi: 10.1242/jcs.262101. Epub 2024 Jul 10. J Cell Sci. 2024. PMID: 38881365 Free PMC article.
-
Lysyl hydroxylase 2-induced collagen cross-link switching promotes metastasis in head and neck squamous cell carcinomas.Neoplasia. 2021 Jun;23(6):594-606. doi: 10.1016/j.neo.2021.05.014. Epub 2021 Jun 6. Neoplasia. 2021. PMID: 34107376 Free PMC article.
References
-
- Desmoulière A., Darby I., Costa A.M., Raccurt M., Tuchweber B., Sommer P., Gabbiani G. Extracellular matrix deposition, lysyl oxidase expression, and myofibroblastic differentiation during the initial stages of cholestatic fibrosis in the rat. Lab. Investig. 1997;76:765–778. - PubMed
-
- Pizzo A.M., Kokini K., Vaughn L.C., Waisner B.Z., Voytik-Harbin S.L. Extracellular matrix (ECM) microstructural composition regulates local cell-ECM biomechanics and fundamental fibroblast behavior: A multidimensional perspective. J. Appl. Physiol. 2005;98:1909–1921. doi: 10.1152/japplphysiol.01137.2004. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

