Micronized sacchachitin promotes satellite cell proliferation through TAK1-JNK-AP-1 signaling pathway predominantly by TLR2 activation
- PMID: 33514380
- PMCID: PMC7510329
- DOI: 10.1186/s13020-020-00381-3
Micronized sacchachitin promotes satellite cell proliferation through TAK1-JNK-AP-1 signaling pathway predominantly by TLR2 activation
Erratum in
-
Correction to: Micronized sacchachitin promotes satellite cell proliferation through TAK1-JNK-AP-1 signaling pathway predominantly by TLR2 activation.Chin Med. 2021 Feb 4;16(1):17. doi: 10.1186/s13020-020-00420-z. Chin Med. 2021. PMID: 33568161 Free PMC article. No abstract available.
Abstract
Background: Ganoderma sp., such as Ganoderma tsugae (GT), play an important role in traditional Chinese medicine. Ganoderma sp. contains several constituents, including Sacacchin, which has recently drawn attention because it can not only enhance the repair of muscle damage but also strengthen the muscle enforcement. Although Ganoderma sp. have a therapeutic effect for neuromuscular disorders, the underlying mechanism remains unclear. This study investigated the effect and underlying molecular mechanism of micronized sacchachitin (mSC) on satellite cells (SCs), which are known as the muscle stem cells.
Methods: The myogenic cells, included SCs (Pax7+) were isolated from tibialis anterior muscles of a healthy rat and were cultured in growth media with different mSC concentrations. For the evaluation of SC proliferation, these cultivated cells were immunostained with Pax7 and bromodeoxyuridine assessed simultaneously. The molecular signal pathway was further investigated by using Western blotting and signal pathway inhibitors.
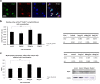
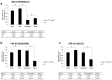
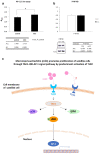
Results: Our data revealed that 200 µg/mL mSC had an optimal capability to significantly enhance the SC proliferation. Furthermore, this enhancement of SC proliferation was verified to be involved with activation of TAK1-JNK-AP-1 signaling pathway through TLR2, whose expression on SC surface was confirmed for the first time here.
Conclusion: Micronized sacchachitin extracted from GT was capable of promoting the proliferation of SC under a correct concentration.
Keywords: MAPK signal pathway; Muscle regeneration; Sacchachitin; Satellite cells; TAK1-JNK-AP-1 signaling pathway.
Conflict of interest statement
The authors report no conflicts of interests in this work.
Figures


References
-
- Pang X, Chen Z, Gao X, Liu W, Slavin M, Yao W, Yu LL. Potential of a novel polysaccharide preparation (GLPP) from Anhui-grown Ganoderma lucidum in tumor treatment and immunostimulation. J Food Sci. 2007;72(6):S435–S442. - PubMed
-
- Su CH, Sun CS, Juan SW, Hu CH, Ke WT, Sheu MT. Fungal mycelia as the source of chitin and polysaccharides and their applications as skin substitutes. Biomaterials. 1997;18(17):1169–1174. - PubMed
-
- Chien RC, Yen MT, Tseng YH, Mau JL. Chemical characteristics and anti-proliferation activities of Ganoderma tsugae polysaccharides. Carbohydr Polym. 2015;128:90–98. - PubMed
-
- Zjawiony JK. Biologically active compounds from Aphyllophorales (polypore) fungi. J Nat Prod. 2004;67(2):300–310. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

