Targeting B7-H3 via chimeric antigen receptor T cells and bispecific killer cell engagers augments antitumor response of cytotoxic lymphocytes
- PMID: 33514401
- PMCID: PMC7844995
- DOI: 10.1186/s13045-020-01024-8
Targeting B7-H3 via chimeric antigen receptor T cells and bispecific killer cell engagers augments antitumor response of cytotoxic lymphocytes
Abstract
Background: B7-H3, an immune-checkpoint molecule and a transmembrane protein, is overexpressed in non-small cell lung cancer (NSCLC), making it an attractive therapeutic target. Here, we aimed to systematically evaluate the value of B7-H3 as a target in NSCLC via T cells expressing B7-H3-specific chimeric antigen receptors (CARs) and bispecific killer cell engager (BiKE)-redirected natural killer (NK) cells.
Methods: We generated B7-H3 CAR and B7-H3/CD16 BiKE derived from an anti-B7-H3 antibody omburtamab that has been shown to preferentially bind tumor tissues and has been safely used in humans in early-phase clinical trials. Antitumor efficacy and induced-immune response of CAR and BiKE were evaluated in vitro and in vivo. The effects of B7-H3 on aerobic glycolysis in NSCLC cells were further investigated.
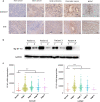
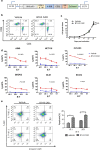
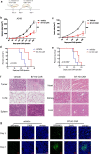
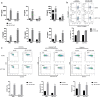
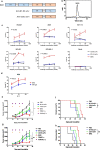
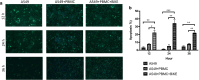
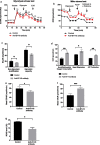
Results: B7-H3 CAR-T cells effectively inhibited NSCLC tumorigenesis in vitro and in vivo. B7-H3 redirection promoted highly specific T-cell infiltration into tumors. Additionally, NK cell activity could be specially triggered by B7-H3/CD16 BiKE through direct CD16 signaling, resulting in significant increase in NK cell activation and target cell death. BiKE improved antitumor efficacy mediated by NK cells in vitro and in vivo, regardless of the cell surface target antigen density on tumor tissues. Furthermore, we found that anti-B7-H3 blockade might alter tumor glucose metabolism via the reactive oxygen species-mediated pathway.
Conclusions: Together, our results suggest that B7-H3 may serve as a target for NSCLC therapy and support the further development of two therapeutic agents in the preclinical and clinical studies.
Keywords: B7-H3; BiKE; Bispecific antibody; CAR T; Chimeric antigen receptor; Non-small cell lung cancer; PD-L1.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
PD-L1 targeting high-affinity NK (t-haNK) cells induce direct antitumor effects and target suppressive MDSC populations.J Immunother Cancer. 2020 May;8(1):e000450. doi: 10.1136/jitc-2019-000450. J Immunother Cancer. 2020. PMID: 32439799 Free PMC article.
-
Chimeric antigen receptor engineered NK cellular immunotherapy overcomes the selection of T-cell escape variant cancer cells.J Immunother Cancer. 2021 Mar;9(3):e002128. doi: 10.1136/jitc-2020-002128. J Immunother Cancer. 2021. PMID: 33741731 Free PMC article.
-
A fc-engineered NKG2D × B7-H3 bispecific antibody enhances the antitumor activity by orchestrating cytotoxic lymphocytes.Int Immunopharmacol. 2025 Aug 28;161:115032. doi: 10.1016/j.intimp.2025.115032. Epub 2025 Jun 13. Int Immunopharmacol. 2025. PMID: 40516256
-
Chimeric antigen receptor (CAR)-T-cell therapy in non-small-cell lung cancer (NSCLC): current status and future perspectives.Cancer Immunol Immunother. 2021 Mar;70(3):619-631. doi: 10.1007/s00262-020-02735-0. Epub 2020 Oct 6. Cancer Immunol Immunother. 2021. PMID: 33025047 Free PMC article. Review.
-
B7-H3-targeted CAR-T cell therapy for solid tumors.Int Rev Immunol. 2022;41(6):625-637. doi: 10.1080/08830185.2022.2102619. Epub 2022 Jul 20. Int Rev Immunol. 2022. PMID: 35855615 Review.
Cited by
-
Therapeutic Targets of Monoclonal Antibodies Used in the Treatment of Cancer: Current and Emerging.Biomedicines. 2023 Jul 24;11(7):2086. doi: 10.3390/biomedicines11072086. Biomedicines. 2023. PMID: 37509725 Free PMC article. Review.
-
Recent Progress in Antibody Epitope Prediction.Antibodies (Basel). 2023 Aug 8;12(3):52. doi: 10.3390/antib12030052. Antibodies (Basel). 2023. PMID: 37606436 Free PMC article. Review.
-
The Application of and Strategy for Gold Nanoparticles in Cancer Immunotherapy.Front Pharmacol. 2021 Jun 7;12:687399. doi: 10.3389/fphar.2021.687399. eCollection 2021. Front Pharmacol. 2021. PMID: 34163367 Free PMC article. Review.
-
Potent Apoptosis Induction by a Novel Trispecific B7-H3xCD16xTIGIT 2+1 Common Light Chain Natural Killer Cell Engager.Molecules. 2024 Mar 4;29(5):1140. doi: 10.3390/molecules29051140. Molecules. 2024. PMID: 38474651 Free PMC article.
-
Optimizing CAR-T cell therapy for solid tumors: current challenges and potential strategies.J Hematol Oncol. 2024 Nov 5;17(1):105. doi: 10.1186/s13045-024-01625-7. J Hematol Oncol. 2024. PMID: 39501358 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

