Patterns of treatment with everolimus exemestane in hormone receptor-positive HER2-negative metastatic breast cancer in the era of targeted therapy
- PMID: 33514405
- PMCID: PMC7844919
- DOI: 10.1186/s13058-021-01394-y
Patterns of treatment with everolimus exemestane in hormone receptor-positive HER2-negative metastatic breast cancer in the era of targeted therapy
Abstract
Background: There is currently no clinical trial data regarding the efficacy of everolimus exemestane (EE) following prior treatment with CDK4/6 inhibitors (CDK4/6i). This study assesses the use and efficacy of everolimus exemestane in patients with metastatic HR+ HER2- breast cancer previously treated with endocrine therapy (ET) or endocrine therapy + CDK4/6i.
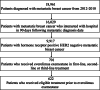
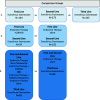
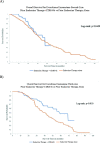
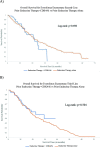
Methods: Retrospective analysis of electronic health record-derived data for HR+ HER2- metastatic breast cancer from 2012 to 2018. The proportion of patients receiving EE first-line, second-line, or third-line, and the median duration of EE prior to next line of treatment (TTNT) by line of therapy was calculated. OS for patients receiving EE first-line, second-line, or third-line, indexed to the date of first-line therapy initiation and stratified by prior treatment received, was calculated with Kaplan-Meier method with multivariable Cox proportional hazards regression analysis.
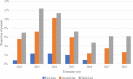
Results: Six hundred twenty-two patients received EE first-line (n = 104, 16.7%), second-line (n = 273, 43.9%) or third-line (n = 245, 39.4%). Median TTNT was 8.3 months, 5.5 months, and 4.8 months respectively. Median TTNT of EE second-line was longer following prior ET alone compared to prior ET + CDK4/6i (6.2 months (95% CI 5.2, 7.3) vs 4.3 months (95% CI 3.2, 5.7) respectively, p = 0.03). Similarly, EE third-line following ET alone vs ET + CDK4/6i in first- or second-line resulted in median TTNT 5.6 months (95% CI 4.4, 6.9) vs 4.1 months (95% CI 3.6, 6.1) respectively, although this was not statistically significant (p = 0.08). Median OS was longer for patients who received EE following prior ET + CDK4/6i. EE second-line following ET + CDK 4/6i vs ET alone resulted in median OS 37.7 months vs. 32.7 months (p = 0.449). EE third-line following ET + CDK4/6i vs prior ET alone resulted in median OS 59.2 months vs. 40.8 months (p < 0.010). This difference in OS was not statistically significant when indexed to the start of EE therapy.
Conclusion: This study suggests that EE remains an effective treatment option after prior ET or ET + CDK4/6i use. Median TTNT of EE was longer for patients who received prior ET, whereas median OS was longer for patients who received prior ET + CDK4/6i. However, this improvement in OS was not statistically significant when indexed to the start of EE therapy suggesting that OS benefit is primarily driven by prior CDK4/6i use. EE remains an effective treatment option regardless of prior treatment option.
Keywords: CDK4/6 inhibitors; Endocrine therapy; Everolimus; Exemestane; Metastatic hormone-positive HER2-negative breast cancer; Sequence of therapy; Targeted therapy.
Conflict of interest statement
L.P. has received consulting fees and honoraria from Seattle Genetics, Pfizer, Astra Zeneca, Merck, Novartis, Bristol Myers Squibb, Pfizer, Genentech, Eisai, Pieris, Immunomedics, Clovis, Syndax, H3Bio, Radius Health, and Daiichi and institutional research funding from Seattle Genetics, AstraZeneca, Merck, Pfizer, and Bristol Myers Squibb. K.A. has received research funding from Genentech and consulting fees/honoria from Genentech, Huron pharmaceuticals, and Celgene and equity from Carrum health. S.M. has received consulting fees from Eisai, Celgene, and Cardinal Health and grant funding from Genentech and Pfizer. M.R. has grant funding from the ASCO Conquer Cancer YIA grant.
Figures
References
-
- DeSantis CE, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;78:6011.
-
- Finn RS, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

