ACE2 Nascence, trafficking, and SARS-CoV-2 pathogenesis: the saga continues
- PMID: 33514423
- PMCID: PMC7844112
- DOI: 10.1186/s40246-021-00304-9
ACE2 Nascence, trafficking, and SARS-CoV-2 pathogenesis: the saga continues
Abstract
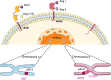
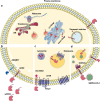
With the emergence of the novel coronavirus SARS-CoV-2 since December 2019, more than 65 million cases have been reported worldwide. This virus has shown high infectivity and severe symptoms in some cases, leading to over 1.5 million deaths globally. Despite the collaborative and concerted research efforts that have been made, no effective medication for COVID-19 (coronavirus disease-2019) is currently available. SARS-CoV-2 uses the angiotensin-converting enzyme 2 (ACE2) as an initial mediator for viral attachment and host cell invasion. ACE2 is widely distributed in the human tissues including the cell surface of lung cells which represent the primary site of the infection. Inhibiting or reducing cell surface availability of ACE2 represents a promising therapy for tackling COVID-19. In this context, most ACE2-based therapeutic strategies have aimed to tackle the virus through the use of angiotensin-converting enzyme (ACE) inhibitors or neutralizing the virus by exogenous administration of ACE2, which does not directly aim to reduce its membrane availability. However, through this review, we present a different perspective focusing on the subcellular localization and trafficking of ACE2. Membrane targeting of ACE2, and shedding and cellular trafficking pathways including the internalization are not well elucidated in literature. Therefore, we hereby present an overview of the fate of newly synthesized ACE2, its post translational modifications, and what is known of its trafficking pathways. In addition, we highlight the possibility that some of the identified ACE2 missense variants might affect its trafficking efficiency and localization and hence may explain some of the observed variable severity of SARS-CoV-2 infections. Moreover, an extensive understanding of these processes is necessarily required to evaluate the potential use of ACE2 as a credible therapeutic target.
Keywords: Angiotensin-converting enzyme 2 (ACE2); COVID-19; Localization; SARS-CoV-2; Trafficking.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Tigerstedt R, Bergman PQ. Niere und Kreislauf1. Skand Arch Für Physiol. 1898;8:223–271. doi: 10.1111/j.1748-1716.1898.tb00272.x. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

