Structural changes in the sacroiliac joint on MRI and relationship to ASDAS inactive disease in axial spondyloarthritis: a 2-year study comparing treatment with etanercept in EMBARK to a contemporary control cohort in DESIR
- PMID: 33514428
- PMCID: PMC7844996
- DOI: 10.1186/s13075-021-02428-8
Structural changes in the sacroiliac joint on MRI and relationship to ASDAS inactive disease in axial spondyloarthritis: a 2-year study comparing treatment with etanercept in EMBARK to a contemporary control cohort in DESIR
Abstract
Background: Limited information is available on the impact of treatment with a tumor necrosis factor inhibitor (TNFi) on structural lesions in patients with recent-onset axial spondyloarthritis (axSpA). We compared 2-year structural lesion changes on magnetic resonance imaging (MRI) in the sacroiliac joints (SIJ) of patients with recent-onset axSpA receiving etanercept in a clinical trial (EMBARK) to similar patients not receiving biologics in a cohort study (DESIR). We also evaluated the relationship between the Ankylosing Spondylitis Disease Activity Score (ASDAS) and change in MRI structural parameters.
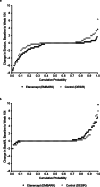
Methods: The difference between etanercept (EMBARK) and control (DESIR) in the net percentage of patients with structural lesion change was determined using the SpondyloArthritis Research Consortium of Canada SIJ Structural Score, with and without adjustment for baseline covariates. The relationship between sustained ASDAS inactive disease, defined as the presence of ASDAS < 1.3 for at least 2 consecutive time points 6 months apart, and structural lesion change was evaluated.
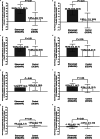
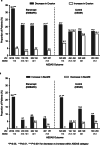
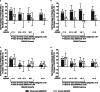
Results: This study included 163 patients from the EMBARK trial and 76 from DESIR. The net percentage of patients with erosion decrease was significantly greater for etanercept vs control: unadjusted: 23.9% vs 5.3%; P = 0.01, adjusted: 23.1% vs 2.9%; P = 0.01. For the patients attaining sustained ASDAS inactive disease on etanercept, erosion decrease was evident in significantly more than erosion increase: 34/104 (32.7%) vs 5/104 (4.8%); P < 0.001. A higher proportion had erosion decrease and backfill increase than patients in other ASDAS status categories. However, the trend across ASDAS categories was not significant and decrease in erosion was observed even in patients without a sustained ASDAS response.
Conclusions: These data show that a greater proportion of patients achieved regression of erosion with versus without etanercept. However, the link between achieving sustained ASDAS inactive disease and structural lesion change on MRI could not be clearly established.
Trial registration: EMBARK: ClinicalTrials.gov identifier: NCT01258738 , Registered 13 December 2010; DESIR: ClinicalTrials.gov identifier: NCT01648907 , Registered 24 July 2012.
Keywords: ASDAS; Anti-TNF; Axial spondyloarthritis; Etanercept; MRI; Sacroiliac joint.
Conflict of interest statement
WPM has received grant/research support from AbbVie, Novartis, and Pfizer, consulted for AbbVie, Boehringer, Celgene, Galapagos, Janssen, Lilly, Merck, Novartis, Pfizer, and UCB, and is the chief medical officer of CaRE Arthritis Ltd. PC has consulted for AbbVie, BMS, Celgene, Janssen, Novartis, Merck, Pfizer, Roche, UCB, and Lilly. MdH is an employee of MdH Research. RGL has consulted for AbbVie, Bioclinica, Janssen, Parexel, and UCB. RL has received grant/research support from AbbVie, Amgen, Centocor, Novartis, Pfizer, Roche, and UCB; consulted for AbbVie, Ablynx, Amgen, AstraZeneca, BMS, Celgene, Janssen, Galapagos, GlaxoSmithKline, Novartis, Novo-Nordisk, Merck, Pfizer, Roche, Schering-Plough, TiGenix, and UCB; and is Director of Rheumatology Consultancy BV. AM has received grant/research support from AbbVie, Pfizer, and UCB and consulted for AbbVie, Janssen, Merck, Novartis, Pfizer, Sanofi, and UCB. DvdH has consulted for AbbVie, Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi, Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda, and UCB, and is the Director of Imaging Rheumatology bv. JFB, HJ, and RP were employees of Pfizer at the time the manuscript was written. BV is an employee of and owns stock in Pfizer. AS is an employee of Syneos Health and was contracted by Pfizer to provide statistical support for the development of this paper. MD has received grant/research support from Pfizer, AbbVie, UCB, Merck, and Lilly and has consulted for Pfizer, AbbVie, UCB, Merck, and Lilly.
No nonfinancial conflict of interest exists for any author.
Figures
Similar articles
-
Evaluation of the change in structural radiographic sacroiliac joint damage after 2 years of etanercept therapy (EMBARK trial) in comparison to a contemporary control cohort (DESIR cohort) in recent onset axial spondyloarthritis.Ann Rheum Dis. 2018 Feb;77(2):221-227. doi: 10.1136/annrheumdis-2017-212008. Epub 2017 Sep 29. Ann Rheum Dis. 2018. PMID: 28970213 Free PMC article. Clinical Trial.
-
Erosions on T1-Weighted Magnetic Resonance Imaging Versus Radiography of Sacroiliac Joints in Recent-Onset Axial Spondyloarthritis: 2-Year Data (EMBARK Trial and DESIR Cohort).J Rheumatol. 2024 May 1;51(5):462-471. doi: 10.3899/jrheum.2023-0906. J Rheumatol. 2024. PMID: 38359938
-
Modification of structural lesions on MRI of the sacroiliac joints by etanercept in the EMBARK trial: a 12-week randomised placebo-controlled trial in patients with non-radiographic axial spondyloarthritis.Ann Rheum Dis. 2018 Jan;77(1):78-84. doi: 10.1136/annrheumdis-2017-211605. Epub 2017 Sep 29. Ann Rheum Dis. 2018. PMID: 28970212 Free PMC article. Clinical Trial.
-
Change in MRI in patients with spondyloarthritis treated with anti-TNF agents: systematic review of the literature and meta-analysis.Clin Exp Rheumatol. 2021 Mar-Apr;39(2):242-252. doi: 10.55563/clinexprheumatol/fsluso. Epub 2021 Jan 21. Clin Exp Rheumatol. 2021. PMID: 33506749
-
Effects of disease-modifying anti-rheumatic drugs on sacroiliac MRI score in axial spondyloarthritis: a systematic review and meta-analysis.Clin Rheumatol. 2024 Mar;43(3):1045-1052. doi: 10.1007/s10067-023-06849-5. Epub 2023 Dec 29. Clin Rheumatol. 2024. PMID: 38158505
Cited by
-
New bone formation at the sacroiliac joint in axial spondyloarthritis: characterization of backfill in MRI and CT.Rheumatology (Oxford). 2023 Dec 1;62(12):3893-3898. doi: 10.1093/rheumatology/kead142. Rheumatology (Oxford). 2023. PMID: 37018132 Free PMC article.
-
Osteoimmunology of Spondyloarthritis.Int J Mol Sci. 2023 Oct 5;24(19):14924. doi: 10.3390/ijms241914924. Int J Mol Sci. 2023. PMID: 37834372 Free PMC article. Review.
-
Active Inflammatory and Chronic Structural Damages of Sacroiliac Joint in Patients With Radiographic Axial Spondyloarthritis and Non-Radiographic Axial Spondyloarthritis.Front Immunol. 2021 Jul 27;12:700260. doi: 10.3389/fimmu.2021.700260. eCollection 2021. Front Immunol. 2021. PMID: 34386008 Free PMC article.
-
New Bone Formation in Axial Spondyloarthritis: A Review.Rofo. 2024 Jun;196(6):550-559. doi: 10.1055/a-2193-1970. Epub 2023 Nov 9. Rofo. 2024. PMID: 37944938 Free PMC article. Review.
-
Management of Axial Spondyloarthritis - Insights into Upadacitinib.Drug Des Devel Ther. 2022 Oct 19;16:3609-3620. doi: 10.2147/DDDT.S330413. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 36268520 Free PMC article. Review.
References
-
- Sepriano A, Regel A, van der Heijde D, Braun J, Baraliakos X, Landewé R, et al. Efficacy and safety of biological and targeted-synthetic DMARDs: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open. 2017;3(1):e000396. doi: 10.1136/rmdopen-2016-000396. - DOI - PMC - PubMed
-
- Corbett M, Soares M, Jhuti G, Rice S, Spackman E, Sideris E, et al. Tumour necrosis factor-α inhibitors for ankylosing spondylitis and non-radiographic axial spondyloarthritis: a systematic review and economic evaluation. Health Technol Assess. 2016;20(9):1–334. doi: 10.3310/hta20090. - DOI - PMC - PubMed
-
- Diekhoff T, Hermann K-GA, Greese J, Schwenke C, Poddubnyy D, Hamm B, et al. Comparison of MRI with radiography for detecting structural lesions of the sacroiliac joint using CT as standard of reference: results from the SIMACT study. Ann Rheum Dis. 2017;76(9):1502–1508. doi: 10.1136/annrheumdis-2016-210640. - DOI - PubMed
-
- Maksymowych WP, Wichuk S, Dougados M, Jones H, Szumski A, Bukowski JF, et al. MRI evidence of structural changes in the sacroiliac joints of patients with non-radiographic axial spondyloarthritis even in the absence of MRI inflammation. Arthritis Res Ther. 2017;19:126. doi: 10.1186/s13075-017-1342-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

