Tick Microbiomes in Neotropical Forest Fragments Are Best Explained by Tick-Associated and Environmental Factors Rather than Host Blood Source
- PMID: 33514519
- PMCID: PMC8091620
- DOI: 10.1128/AEM.02668-20
Tick Microbiomes in Neotropical Forest Fragments Are Best Explained by Tick-Associated and Environmental Factors Rather than Host Blood Source
Erratum in
-
Correction for Kueneman et al., "Tick Microbiomes in Neotropical Forest Fragments Are Best Explained by Tick-Associated and Environmental Factors Rather than Host Blood Source".Appl Environ Microbiol. 2021 Jun 11;87(13):e0077721. doi: 10.1128/AEM.00777-21. Epub 2021 Jun 11. Appl Environ Microbiol. 2021. PMID: 34114899 Free PMC article. No abstract available.
Abstract
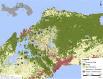
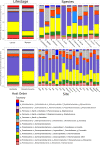
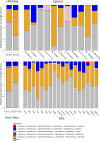
The composition of tick microbiomes varies both within and among tick species. Whether this variation is intrinsic (related to tick characteristics) or extrinsic (related to vertebrate host and habitat) is poorly understood but important, as microbiota can influence the reproductive success and vector competence of ticks. We aimed to uncover what intrinsic and extrinsic factors best explain the microbial composition and taxon richness of 11 species of neotropical ticks collected from eight species of small mammals in 18 forest fragments across central Panama. Microbial richness varied among tick species, life stages, and collection sites but was not related to host blood source. Microbiome composition was best explained by tick life stage, with bacterial assemblages of larvae being a subset of those of nymphs. Collection site explained most of the bacterial taxa with differential abundance across intrinsic and extrinsic factors. Francisella and Rickettsia were highly prevalent, but their proportional abundance differed greatly among tick species, and we found both positive and negative cooccurrence between members of these two genera. Other tick endosymbionts (e.g., Coxiella and Rickettsiella) were associated with specific tick species. In addition, we detected Anaplasma and Bartonella in several tick species. Our results indicate that the microbial composition and richness of neotropical ticks are principally related to intrinsic factors (tick species and life stage) and collection site. Taken together, our analysis informs how tick microbiomes are structured and can help anchor our understanding of tick microbiomes from tropical environments more broadly.IMPORTANCE Blood-feeding arthropod microbiomes often play important roles in disease transmission, yet the factors that structure tick microbial communities in the Neotropics are unknown. Utilizing ticks collected from live animals in neotropical forest fragments, this study teases apart the contributions of intrinsic and extrinsic tick-associated factors on tick microbial composition as well as which specific microbes contribute to differences across tick species, tick life stages, the mammals they fed on, and the locations from where they were sampled. Furthermore, this study provides revelations of how notable tick-associated bacterial genera are interacting with other tick-associated microbes as well as the forest animals they encounter.
Keywords: Lyme disease; Panama; Rickettsia; anaplasma; endosymbionts; environmental microbiology; microbiome; neotropical; tick; tick-borne pathogens.
Copyright © 2021 American Society for Microbiology.
Figures



Similar articles
-
The role of juvenile Dermacentor reticulatus ticks as vectors of microorganisms and the problem of 'meal contamination'.Exp Appl Acarol. 2019 Jun;78(2):181-202. doi: 10.1007/s10493-019-00380-6. Epub 2019 May 22. Exp Appl Acarol. 2019. PMID: 31119415
-
The Microbiome of Ehrlichia-Infected and Uninfected Lone Star Ticks (Amblyomma americanum).PLoS One. 2016 Jan 11;11(1):e0146651. doi: 10.1371/journal.pone.0146651. eCollection 2016. PLoS One. 2016. PMID: 26751816 Free PMC article.
-
Local host-tick coextinction in neotropical forest fragments.Int J Parasitol. 2019 Mar;49(3-4):225-233. doi: 10.1016/j.ijpara.2018.08.008. Epub 2019 Feb 8. Int J Parasitol. 2019. PMID: 30742810
-
The Tick Microbiome: Why Non-pathogenic Microorganisms Matter in Tick Biology and Pathogen Transmission.Front Cell Infect Microbiol. 2017 Jun 8;7:236. doi: 10.3389/fcimb.2017.00236. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28642842 Free PMC article. Review.
-
Update on the intricate tango between tick microbiomes and tick-borne pathogens.Parasite Immunol. 2021 May;43(5):e12813. doi: 10.1111/pim.12813. Epub 2020 Dec 20. Parasite Immunol. 2021. PMID: 33314216 Review.
Cited by
-
Novel Francisella-like endosymbiont and Anaplasma species from Amblyomma nodosum hosted by the anteater Tamandua Mexicana in Mexico.Exp Appl Acarol. 2023 Sep;91(1):111-121. doi: 10.1007/s10493-023-00827-x. Epub 2023 Jul 19. Exp Appl Acarol. 2023. PMID: 37468804
-
The Genetic Diversity of Rickettsiella Symbionts in Ixodes ricinus Throughout Europe.Microb Ecol. 2022 Aug;84(2):613-626. doi: 10.1007/s00248-021-01869-7. Epub 2021 Sep 28. Microb Ecol. 2022. PMID: 34580739 Free PMC article.
-
Microbial diversity of ticks and a novel typhus group Rickettsia species (Rickettsiales bacterium Ac37b) in Inner Mongolia, China.Parasite. 2023;30:58. doi: 10.1051/parasite/2023057. Epub 2023 Dec 12. Parasite. 2023. PMID: 38084939 Free PMC article.
-
16S rRNA metabarcoding for the identification of tick-borne bacteria in ticks in the Republic of Korea.Sci Rep. 2024 Aug 24;14(1):19708. doi: 10.1038/s41598-024-70815-7. Sci Rep. 2024. PMID: 39181959 Free PMC article.
-
Tick-Borne Viruses in a Changing Climate: The Expanding Threat in Africa and Beyond.Microorganisms. 2025 Jun 28;13(7):1509. doi: 10.3390/microorganisms13071509. Microorganisms. 2025. PMID: 40732018 Free PMC article. Review.
References
-
- Medlock JM, Hansford KM, Bormane A, Derdakova M, Estrada-Peña A, George J-C, Golovljova I, Jaenson TGT, Jensen J-K, Jensen PM, Kazimirova M, Oteo JA, Papa A, Pfister K, Plantard O, Randolph SE, Rizzoli A, Santos-Silva MM, Sprong H, Vial L, Hendrickx G, Zeller H, Van Bortel W. 2013. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors 6:1. doi:10.1186/1756-3305-6-1. - DOI - PMC - PubMed
-
- Ergonul O, Whitehouse CA. 2007. Crimean-Congo hemorrhagic fever: a global perspective. Springer Science & Business Media, Dordrecht, the Netherlands.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

