Human physiomimetic model integrating microphysiological systems of the gut, liver, and brain for studies of neurodegenerative diseases
- PMID: 33514545
- PMCID: PMC7846169
- DOI: 10.1126/sciadv.abd1707
Human physiomimetic model integrating microphysiological systems of the gut, liver, and brain for studies of neurodegenerative diseases
Abstract
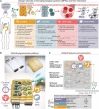
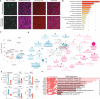
Slow progress in the fight against neurodegenerative diseases (NDs) motivates an urgent need for highly controlled in vitro systems to investigate organ-organ- and organ-immune-specific interactions relevant for disease pathophysiology. Of particular interest is the gut/microbiome-liver-brain axis for parsing out how genetic and environmental factors contribute to NDs. We have developed a mesofluidic platform technology to study gut-liver-cerebral interactions in the context of Parkinson's disease (PD). It connects microphysiological systems (MPSs) of the primary human gut and liver with a human induced pluripotent stem cell-derived cerebral MPS in a systemically circulated common culture medium containing CD4+ regulatory T and T helper 17 cells. We demonstrate this approach using a patient-derived cerebral MPS carrying the PD-causing A53T mutation, gaining two important findings: (i) that systemic interaction enhances features of in vivo-like behavior of cerebral MPSs, and (ii) that microbiome-associated short-chain fatty acids increase expression of pathology-associated pathways in PD.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures




References
-
- Sampson T. R., Debelius J. W., Thron T., Janssen S., Shastri G. G., Ilhan Z. E., Challis C., Schretter C. E., Rocha S., Gradinaru V., Chesselet M.-F., Keshavarzian A., Shannon K. M., Krajmalnik-Brown R., Wittung-Stafshede P., Knight R., Mazmanian S. K., Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480.e12 (2016). - PMC - PubMed
-
- Dalile B., Van Oudenhove L., Vervliet B., Verbeke K., The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478 (2019). - PubMed
-
- Soldner F., Laganière J., Cheng A. W., Hockemeyer D., Gao Q., Alagappan R., Khurana V., Golbe L. I., Myers R. H., Lindquist S., Zhang L., Guschin D., Fong L. K., Vu B. J., Meng X., Urnov F. D., Rebar E. J., Gregory P. D., Zhang H. S., Jaenisch R., Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell 146, 318–331 (2011). - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

