A 3D disease and regeneration model of peripheral nervous system-on-a-chip
- PMID: 33514550
- PMCID: PMC7846159
- DOI: 10.1126/sciadv.abd9749
A 3D disease and regeneration model of peripheral nervous system-on-a-chip
Abstract
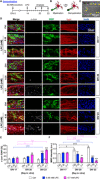
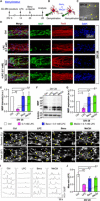
Demyelinating diseases involve loss of myelin sheaths and eventually lead to neurological problems. Unfortunately, the precise mechanisms remain unknown, and there are no effective therapies. To overcome these limitations, a reliable and physiologically relevant in vitro model is required. Here, we present a three-dimensional peripheral nervous system (PNS) microfluidic platform that recapitulates the full spectrum of myelination, demyelination, and remyelination using primary Schwann cells (SCs) and motor neurons (MNs). The platform enables reproducible hydrogel patterning and long-term stable coculture of MNs and SCs over 40 days in vitro based on three distinct design factors. Furthermore, the on-demand detachable substrate allows in-depth biological analysis. We demonstrated the possibility of mimicking segmental demyelination by lysophosphatidylcholine, and recovery of myelin structure by application of two drugs: benzatropine or methylcobalamin. This 3D PNS disease-on-a-chip may serve as a potential platform for understanding the pathophysiology of demyelination and screening drugs for remyelination.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
-
- Mirsky R., Jessen K. R., Schwann cell development, differentiation and myelination. Curr. Opin. Neurobiol. 6, 89–96 (1996). - PubMed
-
- Martin R., Mcfarland H. F., Mcfarlin D. E., Immunological aspects of demyelinating diseases. Annu. Rev. Immunol. 10, 153–187 (1992). - PubMed
-
- Pardo I. D., Rao D. B., Butt M. T., Jortner B. S., Valentine W. M., Arezzo J., Sharma A. K., Bolon B., Toxicologic pathology of the peripheral nervous system (PNS): Overview, challenges, and current practices. Toxicol. Pathol. 46, 1028–1036 (2018). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

