Improper molecular ferroelectrics with simultaneous ultrahigh pyroelectricity and figures of merit
- PMID: 33514555
- PMCID: PMC7846162
- DOI: 10.1126/sciadv.abe3068
Improper molecular ferroelectrics with simultaneous ultrahigh pyroelectricity and figures of merit
Abstract
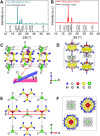
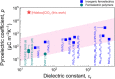
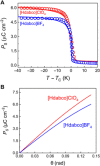
Although ferroelectric materials exhibit large pyroelectric coefficients, their pyroelectric figures of merit (FOMs) are severely limited by their high dielectric constants because of the inverse relationship between FOMs and dielectric constant. Here, we report the molecular ferroelectric [Hdabco]ClO4 and [Hdabco]BF4 (dabco = diazabicyclo[2.2.2]octane) exhibiting improper ferroelectric behavior and pyroelectric FOMs outperforming the current ferroelectrics. Concurrently, the improper molecular ferroelectrics have pyroelectric coefficients that are more than one order of magnitude greater than the state-of-the-art pyroelectric Pb(Mg1/3Nb2/3)O3-PbTiO3 Our first-principles and thermodynamic calculations show that the strong coupling between the order parameters, i.e., the rotation angle of anions and polarization, is responsible for the colossal pyroelectric coefficient of the molecular ferroelectrics. Along with the facile preparation and self-poling features, the improper molecular ferroelectrics hold great promise for high-performance pyroelectric devices.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures

References
-
- Whatmore R. W., Pyroelectric devices and materials. Rep. Prog. Phys. 49, 1335–1386 (1986).
-
- Lang S. B., Pyroelectricity: From ancient curiosity to modern imaging tool. Phys. Today 58, 31–36 (2005).
-
- Felix P., Gamot P., Lacheau P., Raverdy Y., Pyroelectric, dielectric and thermal properties of TGS, DTGS and TGFB. Ferroelectrics 17, 543–551 (1977).
-
- Park J. H., Kim B. K., Song K. H., Park S. J., Electric-field induced strains and pyroelectric coefficients in lead magnesium niobate-lead titanate solid solutions. Mater. Res. Bull. 30, 435–441 (1995).
-
- Kulwicki B. M., Amin A., Beratan H. R., Hanson C. M., Pyroelectric imaging. Proc. IEEE Int. Symp. Appl. Ferroelectrics , 1–10 (1992).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

