Rapid Aging in the Perforant Path Projections to the Rodent Dentate Gyrus
- PMID: 33514675
- PMCID: PMC8018768
- DOI: 10.1523/JNEUROSCI.2376-20.2021
Rapid Aging in the Perforant Path Projections to the Rodent Dentate Gyrus
Abstract
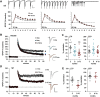
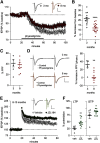
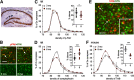
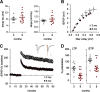
Why layers II/III of entorhinal cortex (EC) deteriorate in advance of other regions during the earliest stages of Alzheimer's disease is poorly understood. Failure of retrograde trophic support from synapses to cell bodies is a common cause of neuronal atrophy, and we accordingly tested for early-life deterioration in projections of rodent layer II EC neurons. Using electrophysiology and quantitative imaging, changes in EC terminals during young adulthood were evaluated in male rats and mice. Field excitatory postsynaptic potentials, input/output curves, and frequency following capacity by lateral perforant path (LPP) projections from lateral EC to dentate gyrus were unchanged from 3 to 8-10 months of age. In contrast, the unusual presynaptic form of long-term potentiation (LTP) expressed by the LPP was profoundly impaired by 8 months in rats and mice. This impairment was accompanied by a reduction in the spine to terminal endocannabinoid signaling needed for LPP-LTP induction and was offset by an agent that enhances signaling. There was a pronounced age-related increase in synaptophysin within LPP terminals, an effect suggestive of incipient pathology. Relatedly, presynaptic levels of TrkB-receptors mediating retrograde trophic signaling-were reduced in the LPP terminal field. LTP and TrkB content were also reduced in the medial perforant path of 8- to 10-month-old rats. As predicted, performance on an LPP-dependent episodic memory task declined by late adulthood. We propose that memory-related synaptic plasticity in EC projections is unusually sensitive to aging, which predisposes EC neurons to pathogenesis later in life.SIGNIFICANCE STATEMENT Neurons within human superficial entorhinal cortex are particularly vulnerable to effects of aging and Alzheimer's disease, although why this is the case is not understood. Here we report that perforant path projections from layer II entorhinal cortex to the dentate gyrus exhibit rapid aging in rodents, including reduced synaptic plasticity and abnormal protein content by 8-10 months of age. Moreover, there was a substantial decline in the performance of an episodic memory task that depends on entorhinal cortical projections at the same ages. Overall, the results suggest that the loss of plasticity and related trophic signaling predispose the entorhinal neurons to functional decline in relatively young adulthood.
Keywords: TrkB; aging; entorhinal cortex; lateral perforant path; long-term potentiation; memory.
Copyright © 2021 the authors.
Figures



References
-
- Andres-Alonso M, Ammar MR, Butnaru I, Gomes GM, Acuña Sanhueza G, Raman R, Yuanxiang PAn, Borgmeyer M, Lopez-Rojas J, Raza SA, Brice N, Hausrat TJ, Macharadze T, Diaz-Gonzalez S, Carlton M, Failla AV, Stork O, Schweizer M, Gundelfinger ED, Kneussel M, et al. (2019) SIPA1L2 controls trafficking and local signaling of TrkB-containing amphisomes at presynaptic terminals. Nat Commun 10:5448. 10.1038/s41467-019-13224-z - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
