Perspectives on skeletal muscle stem cells
- PMID: 33514709
- PMCID: PMC7846784
- DOI: 10.1038/s41467-020-20760-6
Perspectives on skeletal muscle stem cells
Abstract
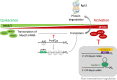
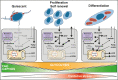
Skeletal muscle has remarkable regeneration capabilities, mainly due to its resident muscle stem cells (MuSCs). In this review, we introduce recently developed technologies and the mechanistic insights they provide to the understanding of MuSC biology, including the re-definition of quiescence and Galert states. Additionally, we present recent studies that link MuSC function with cellular heterogeneity, highlighting the complex regulation of self-renewal in regeneration, muscle disorders and aging. Finally, we discuss MuSC metabolism and its role, as well as the multifaceted regulation of MuSCs by their niche. The presented conceptual advances in the MuSC field impact on our general understanding of stem cells and their therapeutic use in regenerative medicine.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

