Plexin-B2 facilitates glioblastoma infiltration by modulating cell biomechanics
- PMID: 33514835
- PMCID: PMC7846610
- DOI: 10.1038/s42003-021-01667-4
Plexin-B2 facilitates glioblastoma infiltration by modulating cell biomechanics
Abstract
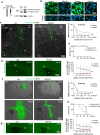
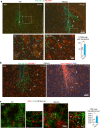
Infiltrative growth is a major cause of high lethality of malignant brain tumors such as glioblastoma (GBM). We show here that GBM cells upregulate guidance receptor Plexin-B2 to gain invasiveness. Deletion of Plexin-B2 in GBM stem cells limited tumor spread and shifted invasion paths from axon fiber tracts to perivascular routes. On a cellular level, Plexin-B2 adjusts cell adhesiveness, migratory responses to different matrix stiffness, and actomyosin dynamics, thus empowering GBM cells to leave stiff tumor bulk and infiltrate softer brain parenchyma. Correspondingly, gene signatures affected by Plexin-B2 were associated with locomotor regulation, matrix interactions, and cellular biomechanics. On a molecular level, the intracellular Ras-GAP domain contributed to Plexin-B2 function, while the signaling relationship with downstream effectors Rap1/2 appeared variable between GBM stem cell lines, reflecting intertumoral heterogeneity. Our studies establish Plexin-B2 as a modulator of cell biomechanics that is usurped by GBM cells to gain invasiveness.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Scherer HJ. Structural development in gliomas. Am. J. Cancer. 1938;34:333–351.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

