A new immunotherapy strategy targeted CD30 in peripheral T-cell lymphomas: CAR-modified T-cell therapy based on CD30 mAb
- PMID: 33514882
- PMCID: PMC8850188
- DOI: 10.1038/s41417-021-00295-8
A new immunotherapy strategy targeted CD30 in peripheral T-cell lymphomas: CAR-modified T-cell therapy based on CD30 mAb
Abstract
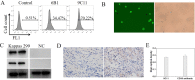
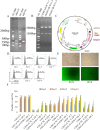
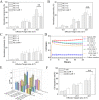
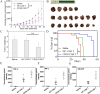
Chimeric antigen receptor T-cell immunotherapy (CAR-T) has shown remarkable efficacy in treating tumors of lymphopoietic origin. Herein, we demonstrate an effective CAR-T cell treatment for recurrent and malignant CD30-positive peripheral T-cell lymphomas (PTCL) has been demonstrated. The extracellular fragment gene sequences of CD30 were obtained from tumor tissues of PTCL patients and cloned into a plasmid vector to express the CD30 antigen. The CD30 targeting single-chain antibody fragment (scFv) was obtained from CD30-positive monoclonal hybridoma cells, which were obtained from CD30 antigen immunized mice. After a second-generation of CAR lentiviral construction, CD30 CAR T cells were produced and used to determine the cytotoxicity of this construct toward Karpas 299 cells. The results of CD30 CAR T-mediated cell lysis show that 9C11-2 CAR T cells could significantly promote the lysis of CD30-positive Karpas 299 cells in both LDH and real-time cell electronic sensing (RTCA) assays. In vivo data show that 9C11-2 CAR T cells effectively suppress the tumor growth in a Karpas 299 cell xenograft NCG mouse model. The CD30 CAR T cells exhibited an efficient cytotoxic effect after being co-cultured with the target cells and they also exhibited a significant tumor-inhibiting ability after being intravenously injected into PTCL xenograft tumors; these observations suggest that the new CD30 CAR-T cell may be a promising therapeutic candidate for cancer therapy.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Blocking CD30 on T Cells by a Dual Specific CAR for CD30 and Colon Cancer Antigens Improves the CAR T Cell Response against CD30- Tumors.Mol Ther. 2019 Oct 2;27(10):1825-1835. doi: 10.1016/j.ymthe.2019.06.007. Epub 2019 Jun 19. Mol Ther. 2019. PMID: 31331813 Free PMC article.
-
[Construction of CD138-targeted chimeric antigen receptor- modified T cells and their effect in multiple myeloma therapy].Zhonghua Xue Ye Xue Za Zhi. 2024 May 14;45(5):436-444. doi: 10.3760/cma.j.cn121090-20240131-00047. Zhonghua Xue Ye Xue Za Zhi. 2024. PMID: 38964917 Free PMC article. Chinese.
-
Challenges of driving CD30-directed CAR-T cells to the clinic.BMC Cancer. 2019 Mar 6;19(1):203. doi: 10.1186/s12885-019-5415-9. BMC Cancer. 2019. PMID: 30841880 Free PMC article. Review.
-
CD30-Redirected Chimeric Antigen Receptor T Cells Target CD30+ and CD30- Embryonal Carcinoma via Antigen-Dependent and Fas/FasL Interactions.Cancer Immunol Res. 2018 Oct;6(10):1274-1287. doi: 10.1158/2326-6066.CIR-18-0065. Epub 2018 Aug 7. Cancer Immunol Res. 2018. PMID: 30087115 Free PMC article.
-
Chimeric Antigen Receptor T Cells in Hodgkin and T-Cell Lymphomas.Hematol Oncol Clin North Am. 2023 Dec;37(6):1107-1124. doi: 10.1016/j.hoc.2023.05.017. Epub 2023 Jun 23. Hematol Oncol Clin North Am. 2023. PMID: 37357070 Free PMC article. Review.
Cited by
-
A structural, genetic and clinical comparison of CAR-T cells and CAR-NK cells: companions or competitors?Front Immunol. 2024 Oct 4;15:1459818. doi: 10.3389/fimmu.2024.1459818. eCollection 2024. Front Immunol. 2024. PMID: 39430751 Free PMC article. Review.
-
Current advances and challenges in CAR T-Cell therapy for solid tumors: tumor-associated antigens and the tumor microenvironment.Exp Hematol Oncol. 2023 Jan 27;12(1):14. doi: 10.1186/s40164-023-00373-7. Exp Hematol Oncol. 2023. PMID: 36707873 Free PMC article. Review.
-
Anti-CD30 antibody-drug conjugate therapy in lymphoma: current knowledge, remaining controversies, and future perspectives.Ann Hematol. 2023 Jan;102(1):13-29. doi: 10.1007/s00277-022-05054-9. Epub 2022 Dec 13. Ann Hematol. 2023. PMID: 36512081 Free PMC article. Review.
-
CAR-T Cell Therapy in Hematological Malignancies: Current Opportunities and Challenges.Front Immunol. 2022 Jun 10;13:927153. doi: 10.3389/fimmu.2022.927153. eCollection 2022. Front Immunol. 2022. PMID: 35757715 Free PMC article. Review.
-
Adoptive Cell Therapy for T-Cell Malignancies.Cancers (Basel). 2022 Dec 23;15(1):94. doi: 10.3390/cancers15010094. Cancers (Basel). 2022. PMID: 36612092 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

