Topical lipoic acid choline ester eye drop for improvement of near visual acuity in subjects with presbyopia: a safety and preliminary efficacy trial
- PMID: 33514891
- PMCID: PMC8602643
- DOI: 10.1038/s41433-020-01391-z
Topical lipoic acid choline ester eye drop for improvement of near visual acuity in subjects with presbyopia: a safety and preliminary efficacy trial
Abstract
Objectives: This study evaluated the safety of topical lipoic acid choline ester (UNR844, 1.5%) ophthalmic solution and its efficacy in improving distance-corrected near visual acuity (DCNVA) in subjects with presbyopia.
Subjects and methods: This was a prospective, randomized, double-masked, and multicentre clinical trial. Subjects with a diagnosis of presbyopia (n = 75) were randomized 2:1 to UNR844 or placebo. On days 1-7, all subjects were dosed unilaterally (twice a day, b.i.d.) in their non-dominant eye to ensure safety and tolerability prior to days 8-91 when dosing was changed to bilateral (b.i.d.). Clinical assessments, including DCNVA and adverse events (AEs), were recorded at each study visit. Patients who completed the study were recruited into a non-interventional follow-up study that monitored them until 7 months after their final UNR844 exposure. The primary endpoints were safety and the mean change in DCNVA from baseline in the study eye.
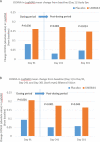
Results: UNR844 administration (n = 50) produced no safety concerns and was well-tolerated, with no clinically-relevant changes in best-corrected distance visual acuity, pupil size, intraocular pressure, or discontinuations due to adverse events. DCNVA improved in the study eye in the UNR844 group compared to placebo during the 91 days of treatment [UNR844 vs. placebo, mean change in LogMAR (SD); -0.159 (0.120) vs. -0.079 (0.116)]. Bilateral DCNVA improved, with 53.1% UNR844 vs. 21.7% placebo subjects gaining ≥10 letters. Improvements in DCNVA were sustained at 5 and 7 months after UNR844 dosing ceased.
Conclusions: These results support further development of UNR844 ophthalmic solution for the treatment of presbyopia.
© 2021. This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply.
Conflict of interest statement
MSK: Consultant—Novartis; Financial support—Novartis and Encore Vision, Inc. SMR: Consultant—Encore Vision, Inc.; Financial support – Encore Vision, Inc. and Novartis. JMS: Consultant – Encore Vision, Inc. DGE: Financial support – Encore Vision, Inc., Allergan and Orasys. KNS: Consultant - Kala Pharmaceuticals, Novaliq, Ocular Therapeutix, Sun Pharma; Financial Support – Encore Vision, Inc., Novartis and Valeant. SV, BC, and MW: - Employees of Novartis. WB: – Employee of Encore Vision, Inc.
Figures


References
-
- Mancil GL, Bailey IL, Brookman KE, Bart Campbell J, Cho MH, Rosenbloom AA, et al. Care of the patient with presbyopia: optometric clinical practice guideline. Am Optom Assoc. 2011.
-
- Milliken CM, Haddad J, Rocha KM. EDOF IOLs: a fresh take on an old concept. MIllennial Eye. 2018. https://millennialeye.com/articles/2018-jan-feb/edof-iols-a-fresh-take-o....
-
- Kondylis G, Klavdianou O, Palioura S. Multifocal and extended depth of focus intraocular lenses. Ann Eye Sci. 2019;4:5. doi: 10.21037/aes.2019.01.01. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

