Lipids, lysosomes and mitochondria: insights into Lewy body formation from rare monogenic disorders
- PMID: 33515275
- PMCID: PMC7952289
- DOI: 10.1007/s00401-021-02266-7
Lipids, lysosomes and mitochondria: insights into Lewy body formation from rare monogenic disorders
Abstract
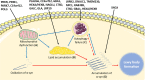
Accumulation of the protein α-synuclein into insoluble intracellular deposits termed Lewy bodies (LBs) is the characteristic neuropathological feature of LB diseases, such as Parkinson's disease (PD), Parkinson's disease dementia (PDD) and dementia with LB (DLB). α-Synuclein aggregation is thought to be a critical pathogenic event in the aetiology of LB disease, based on genetic analyses, fundamental studies using model systems, and the observation of LB pathology in post-mortem tissue. However, some monogenic disorders not traditionally characterised as synucleinopathies, such as lysosomal storage disorders, iron storage disorders and mitochondrial diseases, appear disproportionately vulnerable to the deposition of LBs, perhaps suggesting the process of LB formation may be a result of processes perturbed as a result of these conditions. The present review discusses biological pathways common to monogenic disorders associated with LB formation, identifying catabolic processes, particularly related to lipid homeostasis, autophagy and mitochondrial function, as processes that could contribute to LB formation. These findings are discussed in the context of known mediators of α-synuclein aggregation, highlighting the potential influence of impairments to these processes in the aetiology of LB formation.
Keywords: Alpha-synuclein; Autophagy; Catabolism; Lewy body; Lipid metabolism; Mitochondria.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

